Sensory Rhodopsin-1 (Hs)
Order number: 28911
Description
Sensory rhodopsin-1 (SR-1) is a 7-transmembrane protein that acts as a phototaxis receptor in archaebacteria. Due to its covalently bound colored ligand, SR-1 is ideal for use as a control membrane protein, for example for crystallization assays and biochemical/biophysical experiments. We provide SR-1 of high purity, purified from E.coli as a His-tagged protein.
Datasheets
| Feature | |
|---|---|
| Alternative names | Sensory rhodopsin-1 (SR-1, HsSRI) |
| UniProt Number | P0DMH8 |
| Protein class | 7-transmembrane protein |
| Organism | Halobacterium salinarum S9 |
| Sequence, explained | wild-type sequence 1-234 aa (lacking 15 aa at the C-terminus), 10x His-tag |
| Sequence, One-Letter Code | MDAVATAYLG GAVALIVGVA FVWLLYRSLD GSPHQSALAP LAIIPVFAGL SYVGMAYDIG TVIVNGNQIV GLRYIDWLVT TPILVGYVGY AAGASRRSII GVMVADALMI AVGAGAVVTD GTLKWALFGV SSIFHLSLFA YLYVIFPRVV PDVPEQIGLF NLLKNHIGLL WLAYPLVWLF GPAGIGEATA AGVALTYVFL DVLAKVPYVY FFYARRRVFM HSESHHHHHH HHHH |
| Affinity tags | His-tag (C-terminus) |
| Expression Host | E.coli |
| Size (excluding additional elements) | 234 amino acids; 25,423 Da |
| Buffer composition | 4 M NaCl, 50 mM MES pH 6.0, 0.03% dodecyl maltoside (DDM) |
| Purity | >98%, determined via SDS-PAGE (Figure 1) |
| Purified via | PureCube Ni-NTA Agarose |
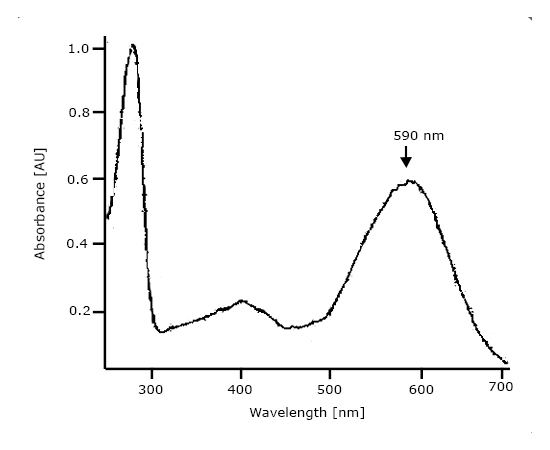
| Absorbance | Extinction coefficient at 560 nm: 63,000 M-1 cm-1. |
| Activity | Binding of ligand all-trans retinal, covalently bound to a lysine residue. Evaluation of UV-VIS spectrum: absorbance ratio 280 nm / 590 nm = 1.7 |
| Function | Photoreceptor protein undergoing a photocycle, Retinal protein, phototaxis receptor |
| Literature references |
|
Lab Results
.jpg)
FAQ
Can I get the datasheet for the Sensory-Rhodopsin-1 protein?
Do you also offer other full-length membrane proteins?
Yes, we do. Just browse through our product protofolio. In case we do not have your desired membrane protein in our store, send a request to our protein services.



