Channelrhodopsin-1 (Ca)
Order number: 28941
Description
Channelrhodopsin 1 is a 7-transmembrane protein that acts as a light-driven cation channel in eukaryotic green algae. Due to its covalently bound colored ligand, it is ideal for use as a control membrane protein, for example for crystallization assays and biochemical/biophysical experiments. We provide channelrhodopsin 1 of high purity, purified from yeast as a His-tagged protein.
Datasheets
| Feature | |
|---|---|
| Alternative names | Channelrhodopsin 1 (CaChR1) |
| UniProt Number | G8HKA1 |
| Protein class | 7-transmembrane protein |
| Organism | Chloromonas augustae (Chlamydomonas augustae) |
| Sequence, explained | wild-type sequence, N-terminal membrane-spanning domain (362 of 715 aa), 10x His-tag |
| Sequence, One-Letter Code | MDTLAWVARE LLSTAHDATP ATATPSTDHS TPSTDHGSGE TFNVTITIGG GHHGGHAGPV DNSIVIGGID GWIAIPAGDC YCAGWYVSHG SSFEATFAHV CQWSIFAVCI LSLLWYAWQY WKATCGWEEV YVCCIELVFI CFELYHEFDS PCSLYLSTAN IVNWLRYSEW LLCCPVILIH LSNVTGLSDD YGRRTMGLLV SDIATIVFGI TAAMLVSWPK IIFYLLGFTM CCYTFYLAAK VLIESFHQVP KGICRHLVKA MAITYYVGWS FFPLIFLFGQ SGFKKISPYA DVIASSFGDL ISKNMFGLLG HFLRVKIHEH ILKHGDIRKT THLRIAGEEK EVETFVEEED EDHHHHHHHH HH |
| Affinity tags | His-tag (C-terminus) |
| Expression Host | Pichia pastoris (yeast) |
| Size | 362 amino acids; 40,661 Da |
| Buffer composition | 100 mM NaCl, 20 mM HEPES pH 7.4, 0.03% dodecyl maltoside (DDM) |
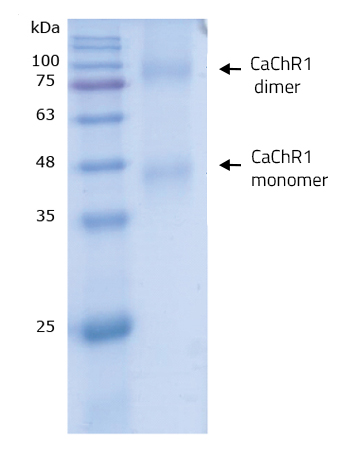
| Purity | >98%, determined via SDS-PAGE (Figure 1) |
| Purified via | PureCube Ni-NTA Agarose |
| Absorbance | Extinction coefficient at 518 nm: 36,000 M-1 cm-1.. Extinction coefficient at 590 nm: 54,000 M-1 cm-1. |
| Activity | Binding of ligand all-trans retinal, covalently bound to a lysine residue. Evaluation of UV-VIS spectrum: absorbance ratio 280 nm / 518 nm = 2.2 (additional vibronic band at 480 nm) |
| Function | Photoreceptor protein undergoing a photocycle, Retinal protein, light-driven cation channel |
| Literature references |
Citations
Lab Results
.jpg)
FAQ
Can I get the datasheet for the Channelodopsin protein?
Do you also offer other full-length membrane proteins?
Yes, we do. Just browse through our product protofolio. In case we do not have your desired membrane protein in our store, send a request to our protein services.