Solving biological structures at sheer cold temperatures – The power of cryo-EM
Bite-size Biotech
Last summer, we expanded our services to include cryogenic electron microscopy (cryo-EM). But what actually is cryo-EM, how does it impact scientific research and what advantages does it have over traditional methods? Let's find out!
Cryo-electron microscopy is a cutting-edge technology that allows researchers to visualize the three-dimensional structure of biological molecules with great detail. It has revolutionized the field of structural biology, enabling scientists to study the structure and function of proteins, viruses, and other biomolecules in ways that were previously more tedious or even impossible.
Cryo-EM works by rapidly freezing samples to cryogenic temperatures, typically around -190°C, in order to preserve their structure in a near-native state. The frozen samples are then imaged in a powerful electron microscope to produce highly detailed – albeit noisy - images of the sample. Thousands of individual images of the sample are then processed using complex algorithms to reconstruct its three-dimensional structure.
Cryo-EM works by rapidly freezing samples to cryogenic temperatures, typically around -190°C, in order to preserve their structure in a near-native state. The frozen samples are then imaged in a powerful electron microscope to produce highly detailed – albeit noisy - images of the sample. Thousands of individual images of the sample are then processed using complex algorithms to reconstruct its three-dimensional structure.
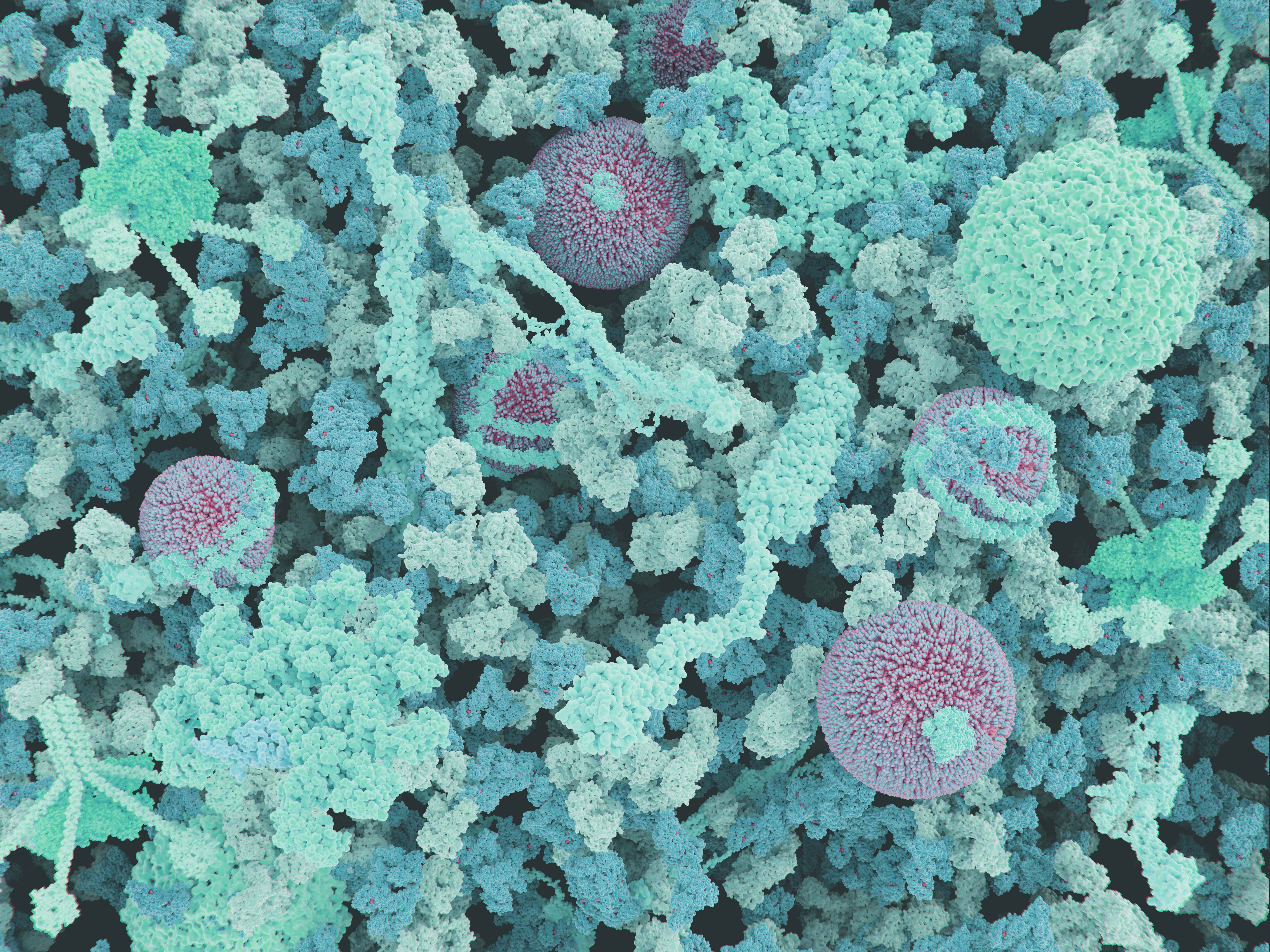
One of the major advantages of cryo-EM is its ability to study large and complex biomolecules that are difficult to crystallize for traditional X-ray crystallography. This has enabled scientists to solve the structures of many important biological molecules, including the Zika virus, various ribosomes from human pathogens, and the HIV-1 envelope glycoprotein. Cryo-EM has also been used to study the structure of protein complexes and their interactions, which has led to a better understanding of the mechanisms of diseases such as cancer and Alzheimer's.
Cryo-EM has rapidly become a popular tool in structural biology, and many advances have been made in recent years. For example, the development of direct electron detectors has greatly improved the signal-to-noise ratio of cryo-EM images. Combined with modern computational image processing methods, this has pushed the limits of what is possible with cryo-EM, allowing researchers to efficiently obtain higher-resolution structures of increasingly smaller, more challenging targets.
In conclusion, cryo-EM is a powerful technology that has profoundly impacted the field of structural biology. Its ability to provide high-resolution structural information on large and complex biomolecules has opened up new avenues for scientific research and has led to a better understanding of the molecular mechanisms of life. As the technology continues to advance, it is likely that cryo-EM will play an even greater role in the future of structural biology.
If you would like to learn more about the exciting possibilities of cryo-EM, please take a look at our more in-depth service video or module six of our in-house Protein Services. There, you can also leave us a message.
Cryo-EM has rapidly become a popular tool in structural biology, and many advances have been made in recent years. For example, the development of direct electron detectors has greatly improved the signal-to-noise ratio of cryo-EM images. Combined with modern computational image processing methods, this has pushed the limits of what is possible with cryo-EM, allowing researchers to efficiently obtain higher-resolution structures of increasingly smaller, more challenging targets.
In conclusion, cryo-EM is a powerful technology that has profoundly impacted the field of structural biology. Its ability to provide high-resolution structural information on large and complex biomolecules has opened up new avenues for scientific research and has led to a better understanding of the molecular mechanisms of life. As the technology continues to advance, it is likely that cryo-EM will play an even greater role in the future of structural biology.
If you would like to learn more about the exciting possibilities of cryo-EM, please take a look at our more in-depth service video or module six of our in-house Protein Services. There, you can also leave us a message.
Sources
- Kuhlbrandt, W. (2014). Cryo-EM enters a new era. eLife, 3, e03678. doi: 10.7554/eLife.03678
- Nogales, E. (2016). The development of cryo-EM into a mainstream structural biology technique. Nature Methods, 13(1), 24-27. doi: 10.1038/nmeth.3685
- Cheng, Y. (2015). Single-particle cryo-EM at crystallographic resolution. Cell. 161(3): 450–457. doi: 10.1016/j.cell.2015.03.049
- Mao Y., Wang L., Gu C., et al. (2013) Molecular architecture of the uncleaved HIV-1 envelope glycoprotein trimer. Proc Natl Acad Sci USA. 110(30):12438-43. doi: 10.1073/pnas.1307382110.
- Siroh D., Chen Z., Sun L., et al. (2016) The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 352(6284):467-470. doi:10.1126/science.aaf5316


