Mechanistic Insights of the Human Sweet Taste Receptors T1R2/T1R3
Paper of the Month - #DIBMA Glycerol & #Rho1D4-Magbeads
In the current study, a collaborative effort led by Shuang Hao and his colleagues at Caltech, published together with partners at Cargill, Cube Biotech, and the Université de Bourgogne-Franche Compté, focused on understanding the binding mechanisms of steviol rebaudiosides on the human sweet taste receptor. Our February paper offers a detailed analysis of binding sites for sweet ligands, providing new insights into the complex mechanisms of human sweet taste receptors.
To ensure that the native lipidic environment of the receptors was preserved, Shuang Hao et al. used tools like DIBMA Glycerol for stabilization and Rho1D4-MagBeads for purification.
To ensure that the native lipidic environment of the receptors was preserved, Shuang Hao et al. used tools like DIBMA Glycerol for stabilization and Rho1D4-MagBeads for purification.
Medical Implication and T1R2/T1R3
The study of sweeteners and their interaction with human taste receptors holds significant industrial and medical implications. These discoveries can be used to increase food sweetness without causing the negative health effects of sugar, like diabetes, obesity, and heart disease. Understanding how different sweeteners interact with taste receptors can guide the design of new compounds that offer the desired sweetness while minimizing health risks.
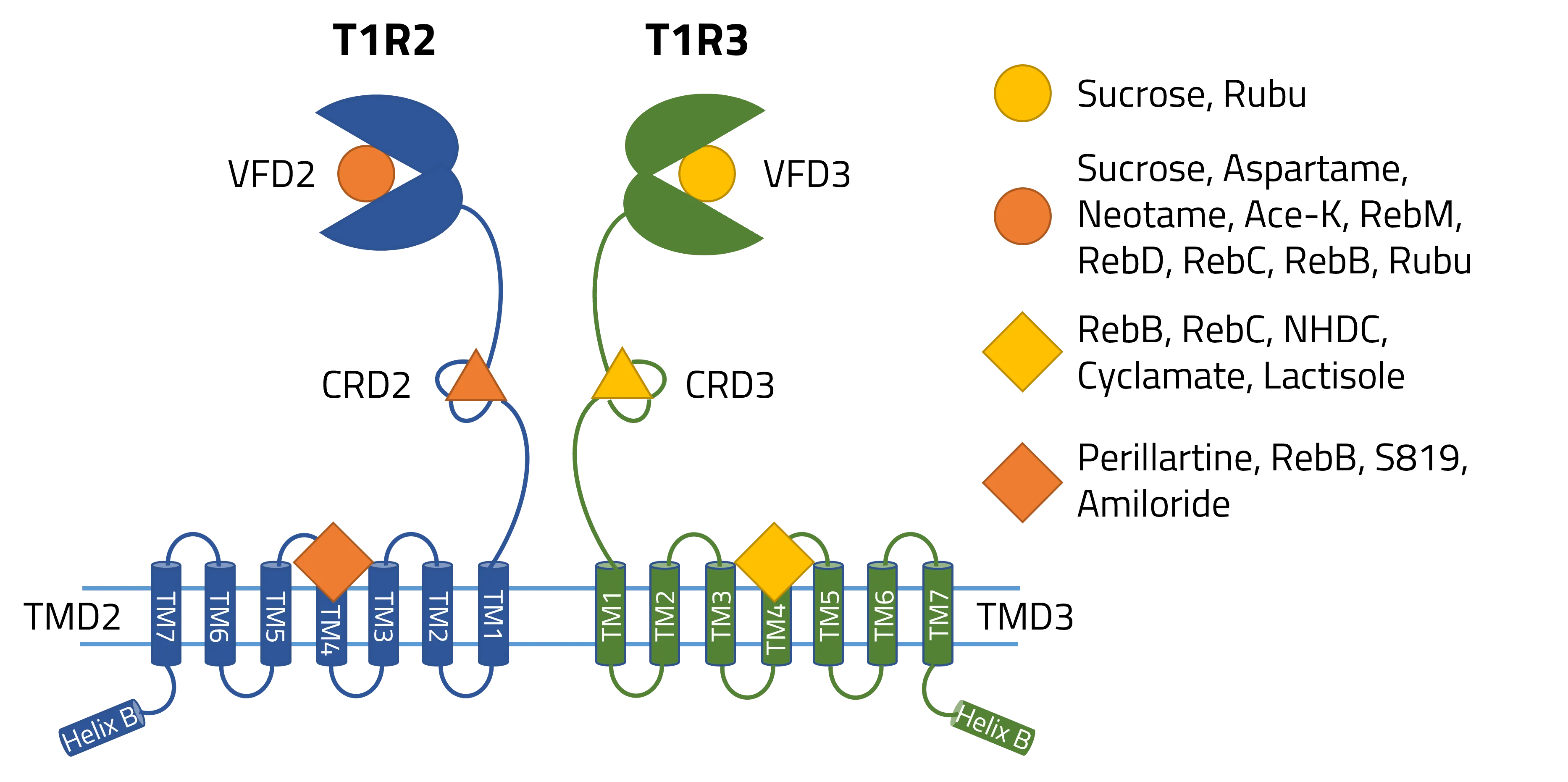
The human sweet taste receptor T1R2/T1R3 is a heterodimer, functioning similarly to other GPCRs by binding ligands and subsequently activating a G protein. Natural sugars like sucrose can bind to the Venus Flytrap Domains (VFD2/VFD3) of T1R2/T1R3, while artificial sweeteners like aspartame only bind to VFD2. These different binding patterns, therefore, significantly influence how we perceive sweetness. Additionally, structural differences between natural and artificial sweeteners affect how they activate the receptor, influencing the intensity and duration of our perceived sweetness.
The human sweet taste receptor T1R2/T1R3 is a heterodimer, functioning similarly to other GPCRs by binding ligands and subsequently activating a G protein. Natural sugars like sucrose can bind to the Venus Flytrap Domains (VFD2/VFD3) of T1R2/T1R3, while artificial sweeteners like aspartame only bind to VFD2. These different binding patterns, therefore, significantly influence how we perceive sweetness. Additionally, structural differences between natural and artificial sweeteners affect how they activate the receptor, influencing the intensity and duration of our perceived sweetness.
Key Results
The study by Hao et al. demonstrates that steviol rebaudiosides can bind to four different sites on the human sweet taste receptor (T1R2/T1R3), which helps explain the varying experimental results. Here are the five key findings:
- The binding of steviol rebaudiosides to multiple sites on the T1R2/T1R3 receptor leads to different signal transduction processes.
- Natural sugars bind to both VFD2 and VFD3, while artificial sweeteners like aspartame bind only to a specific domain.
- G proteins, which are coupled to the TMD2 or TMD3 domain of these receptors, affect the binding affinity of steviol rebaudiosides.
- Experimental and computational methods reveal different binding dynamics of the ligands to the receptor.
- Nine different binding pockets were identified on the human sweet taste receptor.
Discussion & Outlook
The results show that the number and position of the binding sites on the receptor influences sweetness perception. G proteins coupled to different TMD domains can significantly alter the binding affinity for specific ligands, highlighting the importance of analyzing these binding sites to develop new, non-caloric sweeteners. Identifying nine different binding pockets also suggests that the receptor has a high level of plasticity, which could be used to design sweeteners that activate only specific pathways.
In the future, these insights could contribute to creating sweeteners that more closely mimic the taste of sugar while being healthier. For instance, targeting specific binding pockets could lead to sweeteners that provide a more natural taste without the lingering aftertaste often associated with artificial sweeteners. Moreover, understanding the role of G protein coupling in modulating receptor activity could help design compounds with a more balanced sweetness profile, reducing the risk of overstimulation of the taste receptors.
In the future, these insights could contribute to creating sweeteners that more closely mimic the taste of sugar while being healthier. For instance, targeting specific binding pockets could lead to sweeteners that provide a more natural taste without the lingering aftertaste often associated with artificial sweeteners. Moreover, understanding the role of G protein coupling in modulating receptor activity could help design compounds with a more balanced sweetness profile, reducing the risk of overstimulation of the taste receptors.
Next-generation Sweeteners
In summary, the new findings on the binding of steviol rebaudiosides to the T1R2/T1R3 receptor indicate that multiple binding sites greatly influence sweetness perception. The study underscores the complexity of the receptor's interactions with different ligands, suggesting that the traditional view of sweetness as a simple, one-site interaction is incomplete. The combination of experimental and computational approaches used in this study provides a robust framework for future research, potentially leading to breakthroughs in the development of next-generation sweeteners.
Source
Keywords: Human sweet taste receptor; Sweetness perception; Magbeads; DIBMA; Binding dynamics; GPCR


