PureCube Compact Cartridge Ni-NTA
Order number: 31304
Description
The PureCube Ni-NTA Compact cartridge is an IMAC chromatography column prepacked with PureCube Ni-NTA resin. You can choose between two sizes, 1 mL bed volume and 5 mL bed volume. Please have a look at the given features table to ensure that the Cartridge / Column is compatible with your FPLC hardware like ÄKTA TM.
If this is not the case we recommend buying the sole loose PureCube Ni-NTA resin and packing the columns yourself. A dedicated tutorial can be found on the matching site.
Alternatively, we also offer our PureCube Ni-NTA products as magnetic beads, if you prefer this IMAC method.
If this is not the case we recommend buying the sole loose PureCube Ni-NTA resin and packing the columns yourself. A dedicated tutorial can be found on the matching site.
Alternatively, we also offer our PureCube Ni-NTA products as magnetic beads, if you prefer this IMAC method.
Datasheets
| Feature | |
|---|---|
| Usage | Specific binding and purification of 6x His tagged proteins |
| Specificity | Affinity to His tagged proteins |
| Binding capacity | >80 mg/mL |
| Bead Ligand | Ni-NTA |
| Bead size | 40 μm |
| Chelator stability | Stable in buffer containing 10 mM DTT and 1 mM EDTA |
| Filling quantity | Delivered as a 50 % suspension |
| Required equipment & materials |
|
| Feature - Column | ||
|---|---|---|
| PureCube Compact Cartridge 1 mL | PureCube Compact Cartridge 5 mL | |
| Bed Volume | 1 mL | 5 mL |
| Max. Flow Rate | 6 mL/min | 6 mL/min |
| Dimensions: diameter X length (mm) | 5 X 35 | 17 X 35 |
| Body material | Polypropylene | Polypropylene |
| Inlet | 10-32 UNF female thread | 10-32 UNF female thread |
| Outlet | 10-32 UNF female thread | 10-32 UNF female thread |
Citations
Lab Results
High yield and purity
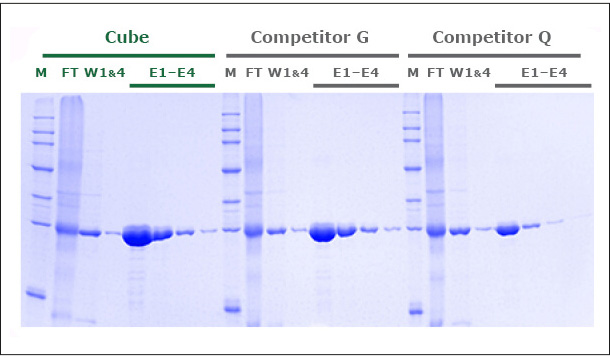
Our unique production process yields a Ni-NTA Agarose that exhibits a protein binding capacity >20% higher than that of two leading competitor products. Figure 1 shows the SDS-PAGE of GFP expressed in E. coli and purified in gravity colums with PureCube Ni-NTA Agarose and the Ni-NTA resin from Competitor G and Competitor Q. The protein yield in 4 elutions (E1-E4, Cube) was 80 mg/mL, compared to 65 and 48 mg/mL obtained with the alternative resins (E1-E4, Competitor G, Competitor Q). Similar results (10-18% higher binding capacity; data not shown here) were obtained comparing the purification of JNK1 (Kinase, 48 kDa) on PureCube Ni-NTA and the Ni-NTA of leading providers.
Our unique production process yields a Ni-NTA Agarose that exhibits a protein binding capacity >20% higher than that of two leading competitor products. Figure 1 shows the SDS-PAGE of GFP expressed in E. coli and purified in gravity colums with PureCube Ni-NTA Agarose and the Ni-NTA resin from Competitor G and Competitor Q. The protein yield in 4 elutions (E1-E4, Cube) was 80 mg/mL, compared to 65 and 48 mg/mL obtained with the alternative resins (E1-E4, Competitor G, Competitor Q). Similar results (10-18% higher binding capacity; data not shown here) were obtained comparing the purification of JNK1 (Kinase, 48 kDa) on PureCube Ni-NTA and the Ni-NTA of leading providers.
Superior DTT and EDTA stability
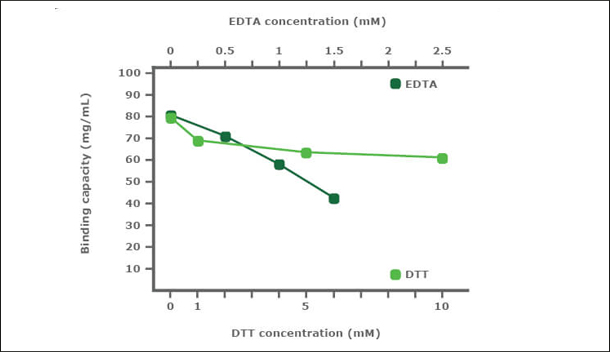
PureCube Ni-NTA Agarose is very robust in the presence of DTT and EDTA. In a stability test, PureCube Ni-NTA Agarose was exposed to increasing concentrations of DTT or EDTA for 1 h. Thereafter, the resins were used to purify E. coli-expressed GFP-His in gravity columns. The binding capacity of the resin decreased in the presence of both DTT and EDTA but the decay rate was shallow. In presence of DTT, PureCube Ni-NTA Agarose lost on average 8% binding capacity with each increase in DTT concentration, resulting in an overall decay of 22% at 10 mM. Even at 1.5 mM EDTA, the resin still exihibits 54% of its maximum binding capacity (Fig. 2).
PureCube Ni-NTA Agarose is very robust in the presence of DTT and EDTA. In a stability test, PureCube Ni-NTA Agarose was exposed to increasing concentrations of DTT or EDTA for 1 h. Thereafter, the resins were used to purify E. coli-expressed GFP-His in gravity columns. The binding capacity of the resin decreased in the presence of both DTT and EDTA but the decay rate was shallow. In presence of DTT, PureCube Ni-NTA Agarose lost on average 8% binding capacity with each increase in DTT concentration, resulting in an overall decay of 22% at 10 mM. Even at 1.5 mM EDTA, the resin still exihibits 54% of its maximum binding capacity (Fig. 2).
Robust against oxidation and regenerable
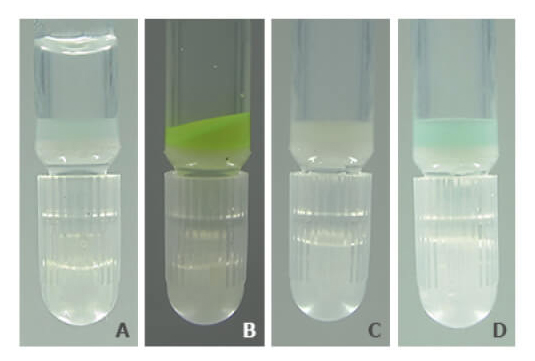
PureCube Ni-NTA Agarose retains its color and function after exposure to as much as 10 mM DTT. Figure 3 shows a photo series of the resin after a 1 h exposure to 5 mM DTT. Unlike other resins, PureCube Ni-NTA Agarose did not turn brown (A). The resin was still able to bind GFP (B), with a measured binding capacity of 65 mg/mL (see Fig. 2). The resin could then be regenerated by stripping the NTA, turning the resin white (C), and reloading it with nickel ions (D). The protocol for regenerating PureCube Ni-NTA Agarose can be downloaded.
PureCube Ni-NTA Agarose retains its color and function after exposure to as much as 10 mM DTT. Figure 3 shows a photo series of the resin after a 1 h exposure to 5 mM DTT. Unlike other resins, PureCube Ni-NTA Agarose did not turn brown (A). The resin was still able to bind GFP (B), with a measured binding capacity of 65 mg/mL (see Fig. 2). The resin could then be regenerated by stripping the NTA, turning the resin white (C), and reloading it with nickel ions (D). The protocol for regenerating PureCube Ni-NTA Agarose can be downloaded.
Video
Video Guide - FPLC
FAQ
Can I get the datasheet for the Ni-NTA COMPACT cartridge?
Is there a difference between a column and a cartridge??
No, these two terms are synonymous.
What are the reasons for nonspecific binding?
Some histidine-rich proteins can also bind to nickel. But washing with NaOH after elution of your protein of interest removes unspecific bound proteins from your resin.
Can Ni-NTA beads be stripped?
Yes, they can. But we recommend using our pre-stripped NTA beads from the beginning:
Can I reuse PureCube Ni-NTA beads or cartridges or can I regenerate them?
Yes, you can reuse the beads! We recommend using our washing & regeneration protocol at least after every 5th use of the beads.



