PureCube Phenyl Agarose
Order number: 39103
Description
Hydrophobic interaction chromatography (HIC) is a reliable method to separate proteins and other biomolecules based on their hydrophobicity. Our PureCube Phenyl agarose resin is a suspension of purification beads for such an approach. If you use HIC with your sample for the first time we recommend testing all of our HIC resins before.
These include:
These include:
| Feature | |
|---|---|
| Usage | As a matriy for Hydrophobic Interaction Chromatography (HIC) |
| Specificity | Hydrophobic biomolecules and proteins |
| Bead size | 100 μm |
| Bead Ligand | Phenyl groups |
| Phenyl group density | 40 µmol/ml |
| BSA binding capacity | 25 mg/ml |
| lysozyme binding capacity | 45 mg/ml |
| pH stability | 2-13 |
| Filling quantity | Delivered as a 50 % suspension |
| Matrix | 7.5% cross-linked agarose |
| Short Term Storage | In neutral buffer at 4°C |
| Long Term Storage | 20 mM sodium acetate, 20% ethanol, pH 6.5 at 4 °C |
| Chemical Structure of Phenyl ligand | 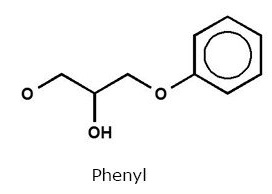 |
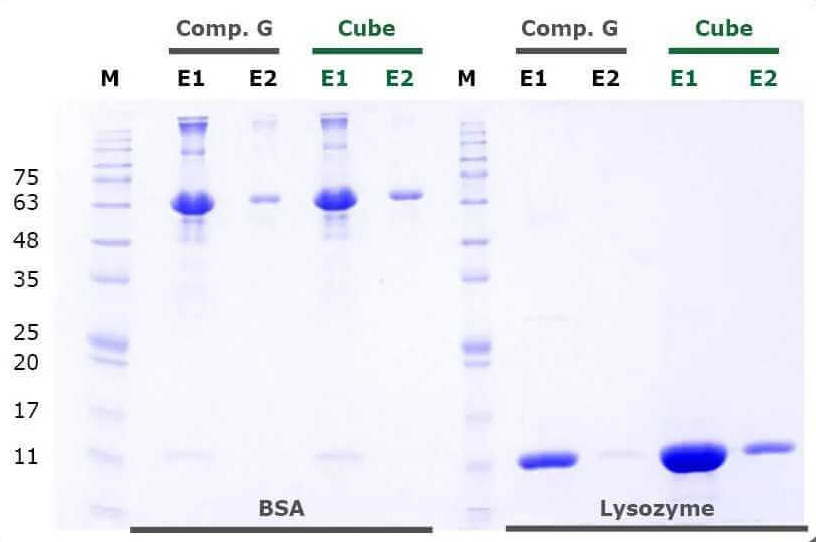
Lab Results
Purification Resins
Hydrophobic interaction chromatography is a well-established method to purify proteins according to their surface charge and hydrophobicity. Chromatography matrices that provide a hydrophobic surface are used for separation. This method does not require any affinity tags to be fused to the protein to be purified. Therefore it is suitable for the purification of both native and recombinant proteins. We offer hydrophobic interaction matrices, with phenyl, octyl, or butyl groups on the surface. For a novel protein, comparing different matrices is strongly recommended, and a HIC screening set with small quantities of each matrix is available. Our PureCube Agaroses are based on BioWorks Workbeads, providing highest binding capacities and reproducible results, especially in FPLC chromatography applications.
Binding
Proteins might precipitate at very high salt concentrations, therefore it is recommended to test in small scale experiments if the protein of interest stays in solution at the salt concentration required for binding.
Elution
Bound proteins can be eluted with low-salt buffers. It is highly recommended to use a salt gradient (e.g. ammonium sulfate or sodium chloride) for elution to ensure that the protein is kept in the optimal salt concentration required for stability.
Hydrophobic interaction chromatography is a well-established method to purify proteins according to their surface charge and hydrophobicity. Chromatography matrices that provide a hydrophobic surface are used for separation. This method does not require any affinity tags to be fused to the protein to be purified. Therefore it is suitable for the purification of both native and recombinant proteins. We offer hydrophobic interaction matrices, with phenyl, octyl, or butyl groups on the surface. For a novel protein, comparing different matrices is strongly recommended, and a HIC screening set with small quantities of each matrix is available. Our PureCube Agaroses are based on BioWorks Workbeads, providing highest binding capacities and reproducible results, especially in FPLC chromatography applications.
Binding
Proteins might precipitate at very high salt concentrations, therefore it is recommended to test in small scale experiments if the protein of interest stays in solution at the salt concentration required for binding.
Elution
Bound proteins can be eluted with low-salt buffers. It is highly recommended to use a salt gradient (e.g. ammonium sulfate or sodium chloride) for elution to ensure that the protein is kept in the optimal salt concentration required for stability.
Video
Video Guide - Hydrophobic Interaction Chromatography (Jump to 8:04)
Video Guide - How to pack FPLC cartridges
FAQ
Can I get the datasheet for the PureCube Phenyl Agarose?
What can I do with these beads?
The beads are meant for Hydrophobic Interaction Chromatography or HIC for short. This purification method separates proteins and other biomolecules based on their hydrophobicity. How the process works in detail can be found in THIS VIDEO GUIDE