The Flag®-Tag - A High Affinity Protein Tag
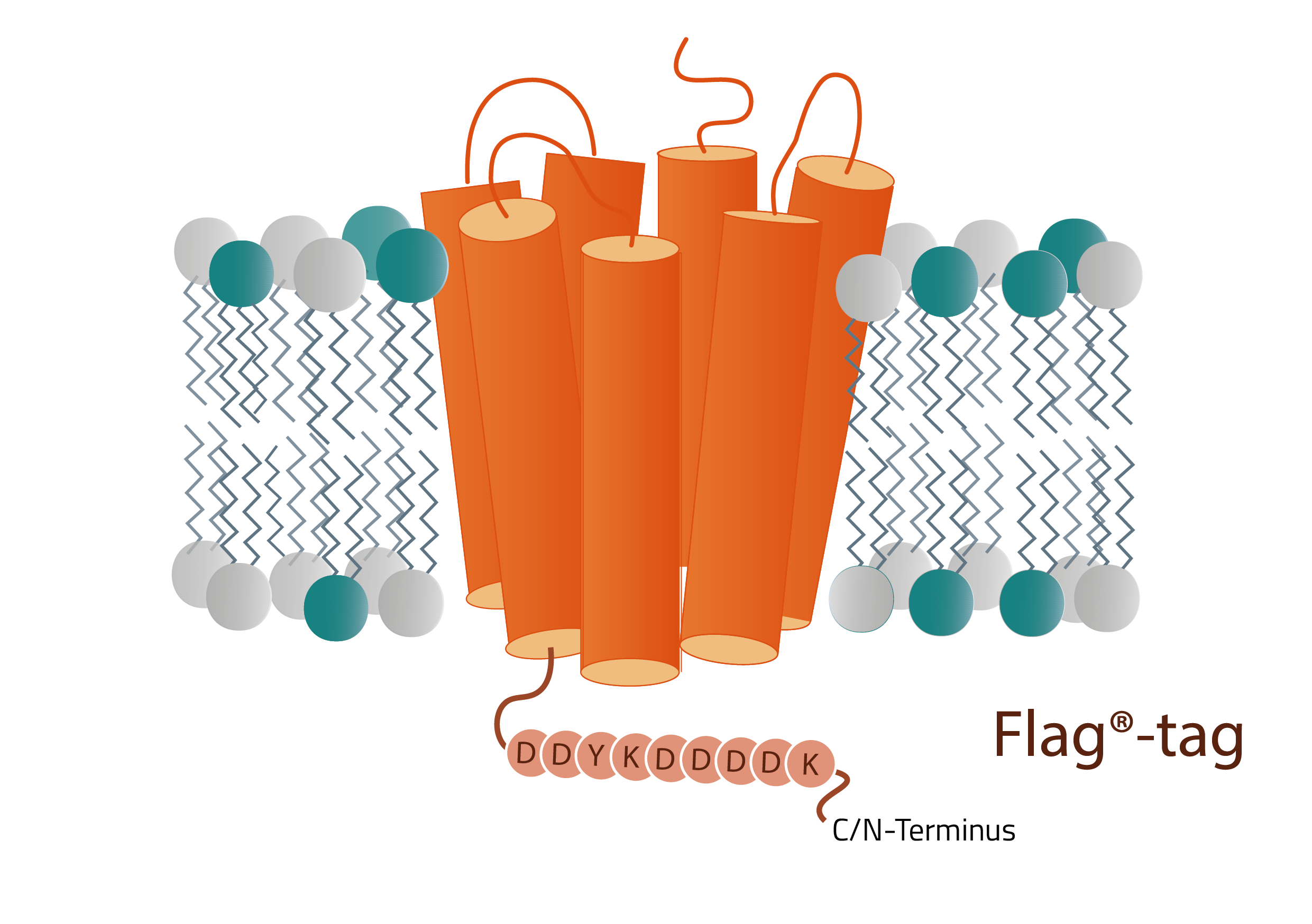
The Flag®-tag, also known as the DYKDDDDK-tag, is a popular protein tag that is commonly used in affinity chromatography and protein research for over 20 years now (6,7,8,9,10,11). As its second name suggests the tag consists of an amino acid sequence DYKDDDDK. (D=Aspartic acid; K=Lysine; Y=Tyrosine). That brings the total size of the tag to 1012.9 dalton or roughly 1 kDa (1). The Flag-tag can be added either to the N-terminus or the C-terminus of a protein (1), respectively. Additionally, it can be implemented in a tandem formation, meaning multiple Flag-tags can be used right next to each other (-DYKDDDDK-DYKDDDDK-DYKDDDDK). A modified version of this tandem formation is even more frequently used today. This altered sequence is DYKDHDG-DYKDHDI-DYKDDDDK (15).
The Flag®-tag is regarded as one of the most specific protein affinity tags out there, but since its development, some good alternatives have also emerged. The Dissociation Constant (KD) of the Flag®-tag sequence to the corresponding antibody is 100 nM (3). The typical protein yield per ml Flag®-tag affinity resin is usually between 0.6-1 mg/ml, which is low, compared to the GST or the Rho1D4-tag. Typically the Flag®-tag is used for protein purification from mammalian expression systems or general immunostaining and immunoprecipitation assays.
Content:
1. The Flag®-tag
2. Alternatives to the Flag®-tag
Table 1: Overview of the most important features of the Flag®-tag
| Features of the FLAG®-tag: | |||
|---|---|---|---|
| Amino acid sequence | DYKDDDDK | ||
| DNA sequence | GAC TAC AAA GAC GAT GAC GAC AAG | ||
| Size | 1012.9 dalton ~ 1 kDa | ||
| Compatibility to recombinant proteins | Can be added either to the N- or C-terminus of a protein | ||
| Can be used in tandem? (Exp: Protein-Flag®- Flag® -Flag® - C-Ter) or as 3xFLAG (15) | Yes | ||
| Combinable with other affinity tags? | Yes | ||
| Specificity of interaction (KD) | 100 nM (3) | ||
| Typical protein yield per ml of high-quality purification resin. | 0.6-1 mg / ml | ||
| Elution conditions | Flag® / DYKDDDDK peptide, pH shift or tag cleavage | ||
| Binds to purification resin / magnetic bead via | DYKDDDDK Flag® antibody (approx. 150 kDa) | ||
| Affinity matrix | Anti-Flag Antibody | ||
Why use the Flag®-tag?
The Flag®-tag can be used for the purification of any recombinant protein fused to the Flag®-tag, using agarose resins or magnetic beads that are coupled to an anti-Flag® antibody. Even though high protein yields cannot be achieved with the Flag®-tag, it is mostly recommended to use the Flag® for membrane protein purification. In this case, purity of the protein is the goal instead of protein yield. High purity can be achieved since the Flag®-tag's affinity is based on an antibody affinity instead of ionic charges as would be the case with His-tag affinity purification using IMAC (4). Additional applications to use the Flag®-tag are as an affinity tag in Western blots and Immunoprecipitation assays.
How to use the Flag®-tag?
As already mentioned the Flag®-tag can be fused either to the C-terminus or the N-terminus of a protein of interest. Which terminus is better suited for the fusion is protein-dependent and needs to be tested for each protein individually. If protein purification is the goal of the project, using the Flag®-tag in tandem is recommended to offer more possible sites for the antibody to attach to. How the actual purification protocol works depends on the purification products that are used and is usually provided by the manufacturer.
2. Alternatives to the Flag®-tag
The Flag®-tag is a famous affinity tag for a reason. Its high specificity outmatched all other tags commonly used by the time of its initial description in 1988 by Hopp et al.(2). And even today this specificity is superior to most affinity tags out there. See our Affinity tag recommendation page for a complete overview. In the meantime between 1988 and today, new discoveries were made and affinity tags developed. The Rho1D4 affinity tag, also called “1D4-tag” is basically like the Flag®-tag, but superior in almost every category. See table 2:
Table 2: Comparison between Rho1D4 and the Flag®-tag
| Feature | Flag®-tag | Rho1D4-tag |
|---|---|---|
| The tag | 8 amino acids (DYKDDDDK) | 9 amino acids (TETSQVAPA) |
| Size of the tag in Da | 1012.0 Da | 902.9 Da |
| Affinity matrix | Anti Flag® antibody (approx. 150 kDa) | Anti-Rho1D4 antibody (approx. 150 kDa) |
| Elution conditions | Flag® peptide, low pH, or tag cleavage | Rho1D4 peptide, low pH, or tag cleavage |
| Specificity of interaction (KD) | 100 nM (3) | 20 nM (5) |
| Typical protein yield per ml resin | 0.6-1 mg/ml | 3-4 mg/ml |
The Rho1D4-tag and its matching antibody were actually discovered 5 years prior to the Flag®-tag in 1983 by Molday and MacKenzie (12). However, its use for protein purification has only been on the rise for the last couple of years (13, 14) which is in clear conjunction with increased demand for purified membrane proteins and the facilitated production thereof.
As table 2 presents, the Rho1D4-tag is superior to the Flag®-tag in every category that is important for protein purification via affinity tag. With its incredible specificity paired with an even higher protein yield, the Rho1D4-tag is the best option for membrane protein purification.

Specificity
The specificity is the Flag®-tag’s strongest feature. Our data here at Cube Biotech, however, suggest that the Rho1D4 antibody’s dissociation constant (KD) is five times higher than the KD of a quality Flag®-tag antibody applying equal conditions. In summary, the specificity of a Rho1D4-tagged protein to its antibody is 5 times higher than that of a Flag®-tagged protein.
Protein Yield
Increased specificity usually corresponds with decreased protein yields when it comes to affinity tag-based protein purification. But not in this case. The average protein yield using the Rho1D4 affinity tag correctly is 3 to 4 mg of protein per 1 ml of used purification resin or magnetic bead. This is 3 to 4 times higher than the highest possible protein yield of Flag®-tag-based protein purification.
3. Example Cloning Strategy - Can also be downloaded as a PDF file
Fusing the Rho1D4-tag or exchanging an established protein tag is easy. Just follow the steps in our short guide and use the primer sequences that we provide. In case you use other restriction sites remember to change the corresponding sequence in the primer.
A good 5' primer for this cloning strategy is made out of three components. In 5' to 3' direction, the first component is a three-nucleotide overhang consisting of "GGG". After this, the restriction site follows. In this example, a NdeI site is shown, but it can be replaced by other sites from the MCS of the used vector. Finally, add approx. ~17 nucleotides of the target gene.
For Copy&Paste: GGG CAT ATG -17nts target gene-
The 3' primer of this cloning recommendation is a little bit more complex: It also starts with a "GGG" spacer, followed by the restriction site. In our example, a XhoI site is shown. After this, two "TCA" stops codons are present to ensure the protein sequence is terminated here. Then comes the actual Rho1D4 sequence "AGCTGGCGCCACCTGGGAAGTCTCGGT". Optionally a linker sequence can be added now. In our experience, a 4 amino acid long linker sequence (Gly-Ser-Ser-Gly) enhances the accessibility of the antibody to the tag. As a linker sequence, we recommend: "GCCGGAGGAGCC". At the 3' end, 20 nucleotides of the reverse antisense target genes follow without the stop codon.
3' primer for Copy&Paste with linker:
GGGCTCGAGTCATCAAGCTGGCGCCACCTGGGAAGTCTCGGTGCCGGAGGAGCC -20nts target gene-
GGGCTCGAGTCATCAAGCTGGCGCCACCTGGGAAGTCTCGGTGCCGGAGGAGCC -20nts target gene-
3' primer for Copy&Paste w/o linker:
GGGCTCGAGTCATCAAGCTGGCGCCACCTGGGAAGTCTCGGT -20nts target gene-
GGGCTCGAGTCATCAAGCTGGCGCCACCTGGGAAGTCTCGGT -20nts target gene-
.jpg)
References:
- Hopp, T. et al. (1988). A Short Polypeptide Marker Sequence Useful for Recombinant Protein Identification and Purification. Bio/Technology. 6. 10.1038/nbt1088-1204
- Einhauer, A. & Jungbauer, A. (2001). The FLAG (TM) peptide, a versatile fusion tag for the purification of recombinant proteins. Journal of biochemical and biophysical methods. 49. 455-65. 10.1016/S0165-022X(01)00213-5.
- Einhauer, A & Jungbauer, A. (2001). Affinity of the monoclonal antibody M1 directed against the FLAG peptide. Journal of chromatography. A. 921. 25-30. 10.1016/S0021-9673(01)00831-7.
- Spriestersbach, Anne & Kubicek, Jan & Schaefer, Frank & Block, Helena & Maertens, Barbara. (2015). Purification of His-Tagged Proteins. Methods in enzymology. 559. 1-15. 10.1016/bs.mie.2014.11.003.
- Cube Biotech PureCube Agarose https://cube-biotech.com/media/pdf/c5/8d/39/331xx-DataSheet-PureCube-Rho1D4-Agarose.pdf
- Koh SS. Et al. (1997). Baculoviral transfer vectors for expression of FLAG fusion proteins in insect cells. BioTechniques ;23:622–7.
- Elhofy A. & Bost KL. (1998) Limitations for purification of murine interleukin-18 when expressed as a fusion protein containing the FLAGe peptide. BioTechniques ;25:425–32.
- Chen J. et al. (1998). A polar octapeptide fused to the N-terminal fusion peptide solubilizes the influenza virus HA2 subunit ectodomain. Biochemistry 1998;37:13643–9
- Humphreys DP. et al. (1999) Efficient site specific removal ` X of a C-terminal FLAG fusion from a Fab using copper II ion catalysed protein cleavage. Protein Eng Ž . 1999;12:179–84.
- Swayden, M. et al. (2019). Profiling Ubiquitin and Ubiquitin-like Dependent Post-translational Modifications and Identification of Significant Alterations. Journal of Visualized Experiments. 10.3791/60402.
- Khoshnejad, M.Vladimir. (2019). CRISPR/Cas9-Mediated Genetic Engineering of Hybridomas for Creation of Antibodies that Allow for Site-Specific Conjugation. 10.1007/978-1-4939-9654-4_7.
- Molday R.S. & MacKanzie D. (1984). Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. 10.1007/978-1-4939-9654-4_7.
- Mattle D. et al.(2015). Mammalian Expression, Purification, and Crystallization of Rhodopsin Variants. In: Jastrzebska B. (eds) Rhodopsin. Methods in Molecular Biology, vol 1271. Humana Press, New York, NY
- Brosig, A. et al (2019). The Axonal Membrane Protein PRG2 Inhibits PTEN and Directs Growth to Branches. Cell Reports. 29. 2028-2040.e8. 10.1016/j.celrep.2019.10.039.
- Ueda M. et al. (2011). The high performance of 3XFLAG for target purification of a bioactive metabolite: A tag combined with a highly effective linker structure. Bioorganic & medicinal chemistry letters. 21. 1359-62. 10.1016/j.bmcl.2011.01.038.
Flag® and Flag-tag® are trademarks of Sigma-Aldrich.


