What Is Rho1D4? - An Affinity Tag Specialized For Membrane Proteins

What is the Rho1D4-tag?
Protein affinity epitope tags find use in numerous applications in life sciences. Ranging from Western Blot detection, protein localization, ELISA assays, and most importantly protein purification. The possible uses for epitope tags are numerous (Molday & Molday 2014).
Rho1D4 or sometimes also just called "1D4-tag" is an epitope tag that originates from the C-terminal end of bovine rhodopsin. Its nine amino acids long sequence is TETSQVAPA, which gives it a size of 902.9 Dalton. The affinity tag and the specific monoclonal antibodies were discovered in 1984 by Hodges et al. and MacKenzie et al.
Rho1D4 or sometimes also just called "1D4-tag" is an epitope tag that originates from the C-terminal end of bovine rhodopsin. Its nine amino acids long sequence is TETSQVAPA, which gives it a size of 902.9 Dalton. The affinity tag and the specific monoclonal antibodies were discovered in 1984 by Hodges et al. and MacKenzie et al.
What are the Advantages of the Rho1D4-tag for Protein Purification?
When choosing an affinity/epitope tag for protein purification the task boils down to two questions:
- Do I need more protein or more purity?
- Does this affinity tag influence the protein's function?
Therefore we recommend using the Rho1D4 tag, especially for the purification of proteins with low abundance. Since these proteins must be purified out of a magnitude of other proteins. Therefore the specificity of the purification bead to the affinity tag must be as high as possible to avoid any unspecific bindings. Something that a His-tag-based purification tends to. The binding of monoclonal Rho1D4 antibodies on the beads is the reason for this specificity. This possibility for maximum protein purity makes the Rho1D4 tag the best option to purify a whole group of low abundance proteins: membrane proteins.
Rho1D4 as an affinity tag for membrane proteins
Membrane proteins are more challenging to work with than their soluble counterparts. The fact that the removal from the cell membrane leaves them unstable without the use of detergents or nanodiscs makes some scientists averse to working with them. Due to the additional fact that more commonly used affinity tags are unfit for proper membrane protein purification further increases this effect. However, there are studies and publications that went ahead and embraced the advantages of the Rho1D4 system and successfully purified active membrane protein, see table 1.
Table 1: Usage of the Rho1D4 system in the literature
| Protein | Expression System | Purity | Yield (total protein recovered) | Purification steps taken |
|---|---|---|---|---|
| hCB2 G protein-coupled receptorLocatelli-Hoops et al. | E.coli | 90% | 4.5 mg/5 L culture | Rho1D4 IAC + IMAC |
| hVN1R1 G protein-coupled receptorCorin et al. | Inducible HEK293S cell line (mammal) | 90% | 1 mg/1 g cells | Rho1D4 IAC + SEC |
| FPR3 G protein-coupled receptorWang & Zhang | Inducible HEK293S cell line (mammal) | 90% | 2 mg/6 g cells | Rho1D4 IAC + SEC |
| TAAR5 G protein-coupled receptorWang et al. | Inducible HEK293S cell line (mammal) | 90% | 1 mg/9 g cells | Rho1D4 IAC + SEC |
| OR131-2 G protein-coupled receptorLeck et al. | Inducible HEK293S cell line (mammal) | 90% | 2.9 mg/10 g cells | Rho1D4 IAC + centrifugation |
| hOR17-4 G protein-coupled receptorCook et al. | Inducible HEK293S cell line (mammal) | 90% | 7.5 mg/2.5 L culture | Rho1D4 IAC + SEC |
| hOR17-4 G protein-coupled receptorCook et al. | Cell-free wheat germ extract | 70%* | 0.3 mg/mL reaction solution* | Rho1D4 IAC + SEC |
| CD81 Tetraspanin membrane proteinTakayama et al. | Inducible HEK293S cell line (mammal) | >95% | 26 ug/3X10^7 cells | Rho1D4 IAC |
| AE1 Solute carrierBonar & Casey | S. cerevisiae strain BJ5457 | 93% | 2.5 mg/18 L culture | Rho1D4 IAC |
Examples of purity and yield reported in the literature for membrane proteins purified with the rho1D4 system. Although many of the proteins purified with the Rho1D4 system have been G protein-coupled receptors, the system has the flexibility to facilitate the characterization of other membrane proteins such as transporters. Referenced articles are listed in the literature cited. IAC: immunoaffinity chromatography; IMAC: immobilized metal affinity chromatography; SEC: size-exclusion chromatography.
Features of the Rho1D4-tag
We compiled the most important data regarding the Rho1D4-tag and its capabilities for protein purification.
Table 2: Highlights of the Rho1D4-tag
| Category | Feature |
|---|---|
| Sequence (Amino Acids) | 9 amino acids: TETSQVAPA |
| Sequence (DNA) | 5'direction: ACC-GAG-ACT-TCC-CAG-GTC-GCG-CCA-GCT 3'direction: AGC-TGG-CGC-GAC-CTG-GGA-AGT-CTC-GGT |
| Size of the tag in Da | 902.9 Da |
| Affinity matrix | Anti-Rho1D4 antibody (approx. 150 kDa) |
| Elution conditions | Rho1D4 peptide, low pH, or tag cleavage |
| Specificity of interaction (KD) | 20 nM |
| Typical protein yield per ml resin | 3-4 mg/ml |
How to add/clone a Rho1D4 Epitope Tag
The best way to add a Rho1D4-tag to your protein of interest is through a PCR strategy that adds the Rho1D4-tag to the C-terminus of the protein of interest. We at Cube Biotech recommend a fairly standard cloning strategy here, as only a few nucleotides need to be added to the gene. We can also give you an example of what such primers may look like.
1. Forward primer: Our primer design assumes that you are using a standard expression vector that includes some form of multiple cloning site (MCS). From the 5' to the 3' end of the primer:
1. Forward primer: Our primer design assumes that you are using a standard expression vector that includes some form of multiple cloning site (MCS). From the 5' to the 3' end of the primer:
- A few overhang nucleotides ease the restriction digest. We suggest 3-4 nts here, but in theory, it could be as many as you want.
- The restriction site. Of course, this depends on your chosen expression vector and its specific MSC. Our example primer uses NdeI.
- 20-25 nts of the N-terminus of your protein of interest. Our example here uses eGFP.
2. Reverse primer: The Reverse primer is longer than the forward primer, as it has to include more features than it. From The 5' to the 3' end of the primer:
- A few overhang nucleotides ease the restriction digest. We suggest 3-4 nts here, but in theory, it could be as many as you want.
- The reverse primer restriction site. Depends on your chosen expression vector and its specific MSC. Our example primer uses XhoI.
- Two TCA stop codons to ensure that the recombinant protein is indeed terminated here
- The Rho1D4-tag
- Optional: A four amino acid long spacer sequence to ensure tag accessibility for the Purification beads.
- 20-25 nts of the 3' end of the gene of interest. NOT including the stop codon. Our example primer uses the eGFP reserve sequence.
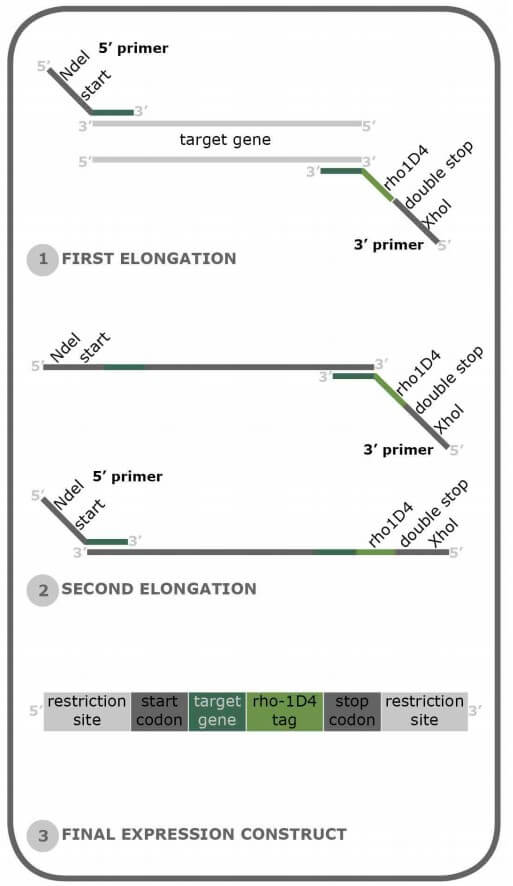
Legend - Forward primer:
5' - Overhang - Restriction site ⮕ NdeI in this case - First 20 nts of eGFP - 3'
5' - GGG CATATG ATGAAACATCACCATCACCA - 3'
5' - Overhang - Restriction site ⮕ NdeI in this case - First 20 nts of eGFP - 3'
5' - GGG CATATG ATGAAACATCACCATCACCA - 3'
Legend - Reverse primer:
5' - Overhang- XhoI site - Stop Codons - Rho1D4-tag - Spacer sequence (optional) - Last 20 nts of eGFP-3'
5' - GGG CTCGAG TCATCA AGCTGGCGCCACCTGGGAAGTCTCGGT GCCGGAGGAGCC TTTGTATAGTTCATCCATGC -3'
Protein Purification using the Rho1D4-tag
Protein purification with the Rho1D4-tag belongs to the affinity chromatography methods. It works by binding the Rho1D4 antibody to either agarose resin or magnetic beads. The purification procedure is separated into the three subsequent parts.
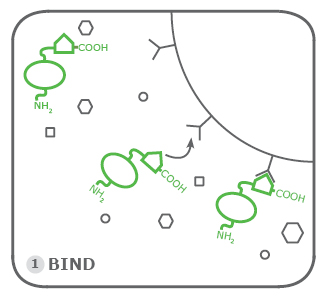
1. Binding:
The first step is binding the Rho1D4-tagged proteins to the agarose resin beads or magnetic beads. This occurs after the cell pellets that contain the tagged proteins underwent cell lysis. This step occurs in a specialized Rho Binding Buffer. Its composition can be found here. Since the Rho1D4-tag is meant for low-expressed proteins and membrane proteins we recommend performing the binding step for multiple hours. At best overnight at room temperature.
The first step is binding the Rho1D4-tagged proteins to the agarose resin beads or magnetic beads. This occurs after the cell pellets that contain the tagged proteins underwent cell lysis. This step occurs in a specialized Rho Binding Buffer. Its composition can be found here. Since the Rho1D4-tag is meant for low-expressed proteins and membrane proteins we recommend performing the binding step for multiple hours. At best overnight at room temperature.
2. Washing:
Now it is time to remove the rest of the cell lysate. This is the actual purification step in this purification assay. Again, the fact that low expressed proteins and membrane proteins are best suited for Rho1D4-tagging comes into play here. Due to the normally low abundance of Rho1D4-tagged proteins, the washing must be performed conscientiously and well. Remaining impurities due to the lack of proper washing can overlap the signal of Rho1D4 tagged protein due to their low abundance. This is avoided by carrying out more washing steps than minimally recommended. At least 2-3 times.
Now it is time to remove the rest of the cell lysate. This is the actual purification step in this purification assay. Again, the fact that low expressed proteins and membrane proteins are best suited for Rho1D4-tagging comes into play here. Due to the normally low abundance of Rho1D4-tagged proteins, the washing must be performed conscientiously and well. Remaining impurities due to the lack of proper washing can overlap the signal of Rho1D4 tagged protein due to their low abundance. This is avoided by carrying out more washing steps than minimally recommended. At least 2-3 times.
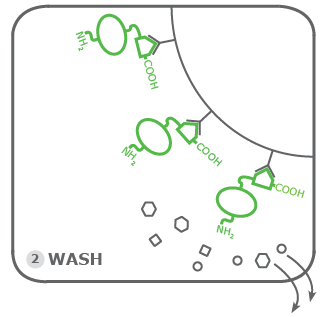
2. Washing:
Now it is time to remove the rest of the cell lysate. This is the actual purification step in this purification assay. Again, the fact that low expressed proteins and membrane proteins are best suited for Rho1D4-tagging comes into play here. Due to the normally low abundance of Rho1D4-tagged proteins, the washing must be performed conscientiously and well. Remaining impurities due to the lack of proper washing can overlap the signal of Rho1D4 tagged protein due to their low abundance. This is avoided by carrying out more washing steps than minimally recommended. At least 2-3 times.
Now it is time to remove the rest of the cell lysate. This is the actual purification step in this purification assay. Again, the fact that low expressed proteins and membrane proteins are best suited for Rho1D4-tagging comes into play here. Due to the normally low abundance of Rho1D4-tagged proteins, the washing must be performed conscientiously and well. Remaining impurities due to the lack of proper washing can overlap the signal of Rho1D4 tagged protein due to their low abundance. This is avoided by carrying out more washing steps than minimally recommended. At least 2-3 times.
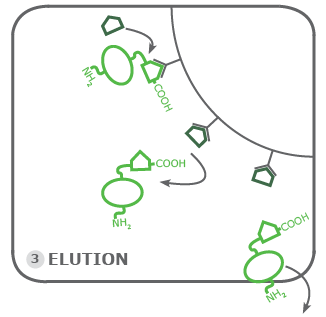
3. Elution:
The final step of the purification is based on the principle of competitive binding of molecules to an affinity target. In this case, the binding of the Rho1D4-tagged proteins is challenged through an excessive amount of Rho1D4 peptide. A magnitude of pure Rho1D4-tags if you may. These peptides first bind to the remaining empty Rho1D4 antibodies on the beads. Then the peptide starts to replace the Rho1D4-tagged protein on the beads, this is the elution. Therefore the concentration of the peptide must not be too low. We recommend at least 200 µmol. The eluted protein is collected inside our elution buffer and is ready to be further processed depending on your planned experiments.
The final step of the purification is based on the principle of competitive binding of molecules to an affinity target. In this case, the binding of the Rho1D4-tagged proteins is challenged through an excessive amount of Rho1D4 peptide. A magnitude of pure Rho1D4-tags if you may. These peptides first bind to the remaining empty Rho1D4 antibodies on the beads. Then the peptide starts to replace the Rho1D4-tagged protein on the beads, this is the elution. Therefore the concentration of the peptide must not be too low. We recommend at least 200 µmol. The eluted protein is collected inside our elution buffer and is ready to be further processed depending on your planned experiments.
Our Product portfolio regarding Rho1D4-tag affinity products has two main protocols that most of these products follow. The only exception are the cartridges, their protocols are depended on the used FPLC hardware.
References
- Molday, L. L. & Molday, R. S. 1D4: a versatile epitope tag for the purification and characterization of expressed membrane and soluble proteins. Methods Mol Biol 1177, 1–15 (2014).
- Hodges, R., Heaton, R., Parker, J. M. R., Molday, L. & Molday, R. Antigen-antibody interaction. Synthetic peptides define linear antigenic determinants recognized by monoclonal antibodies directed to the cytoplasmic carboxyl terminus of rhodopsin. The Journal of biological chemistry 263, 11768–75 (1988).
- Locatelli-Hoops, S. C., Gorshkova, I., Gawrisch, K. & Yeliseev, A. A. Expression, surface immobilization, and characterization of functional recombinant cannabinoid receptor CB2. Biochim Biophys Acta 1834, 2045–2056 (2013).
- Takayama, H., Chelikani, P., Reeves, P., Zhang, S. & Khorana, H. High-Level Expression, Single-Step Immunoaffinity Purification and Characterization of Human Tetraspanin Membrane Protein CD81. PloS one 3, e2314 (2008).
- Cook, B. L. et al. Large-scale production and study of a synthetic G protein-coupled receptor: Human olfactory receptor 17-4. Proc Natl Acad Sci USA 106, 11925 (2009).
- MacKenzie, D., Arendt, A., Hargrave, P., McDowell, J. & Molday, R. Localization of binding sites for carboxyl terminal specific anti-rhodopsin monoclonal antibodies using synthetic peptides. Biochemistry 23, 6544–6549 (1984).
- Wang, X. & Zhang, S. Production of a Bioengineered G-Protein Coupled Receptor of Human Formyl Peptide Receptor 3. PLOS ONE 6, e23076 (2011).
- Bonar, P. & Casey, J. Purification of functional human Cl-/HCO3 - exchanger, AE1, over-expressed in Saccharomyces cerevisiae. Protein expression and purification 74, 106–15 (2010).
- Corin, K. et al. Structure and function analyses of the purified GPCR human vomeronasal type 1 receptor 1. Scientific Reports 1, 172 (2011).
- Cook, B., Tourle, K., Chung, H. & Zhang, S. Study of a Synthetic Human Olfactory Receptor 17-4: Expression and Purification from an Inducible Mammalian Cell Line. PloS one 3, e2920 (2008).
- Leck, K., Zhang, S. & Hauser, C. Study of Bioengineered Zebra Fish Olfactory Receptor 131-2: Receptor Purification and Secondary Structure Analysis. PloS one 5, e15027 (2010).
- Wang, X., Corin, K., Rich, C. & Zhang, S. Study of two G-protein coupled receptor variants of human trace amine-associated receptor 5. Scientific Reports 1, 102 (2011).


