A new generation of Amphipols is here.
Now you can solubilize AND stabilize your membrane proteins with them.
Membrane proteins play a key role in cellular growth processes, in signaling or homeostasis, and they make up a large proportion of medical and therapeutic targets. Nevertheless, it is difficult to extract them from the cell membrane, and the need for a solubilizer or detergent makes it difficult to study their functional or structural properties. Although the classic amphipols were known to be very effective for cryo-EM and mass spectrometry, their reliance on initial detergent extraction limited their potential use. Therefore, the circumvention of this central problem with amphipols would be a great advantage for membrane protein science (Higgins, Flynn, Marconnet et. al, 2021 and Marconnet et. al, 2022).
What's New?
Ultrasolute™ Amphipols now differ from the old Amphipols mainly by the replacement of linear octyl chains for hydrophobic, saturated cycles (Marconnet, Michon and Le Bon et al., 2020). Due to this change, the polymer remarkably improves its ability to extract membrane proteins from a biological membrane, regardless of the membrane and the protein of interest, while maintaining the native structure of the target (Laboratory of Physical and Chemical Biology of Membrane Proteins, Université Paris Diderot).
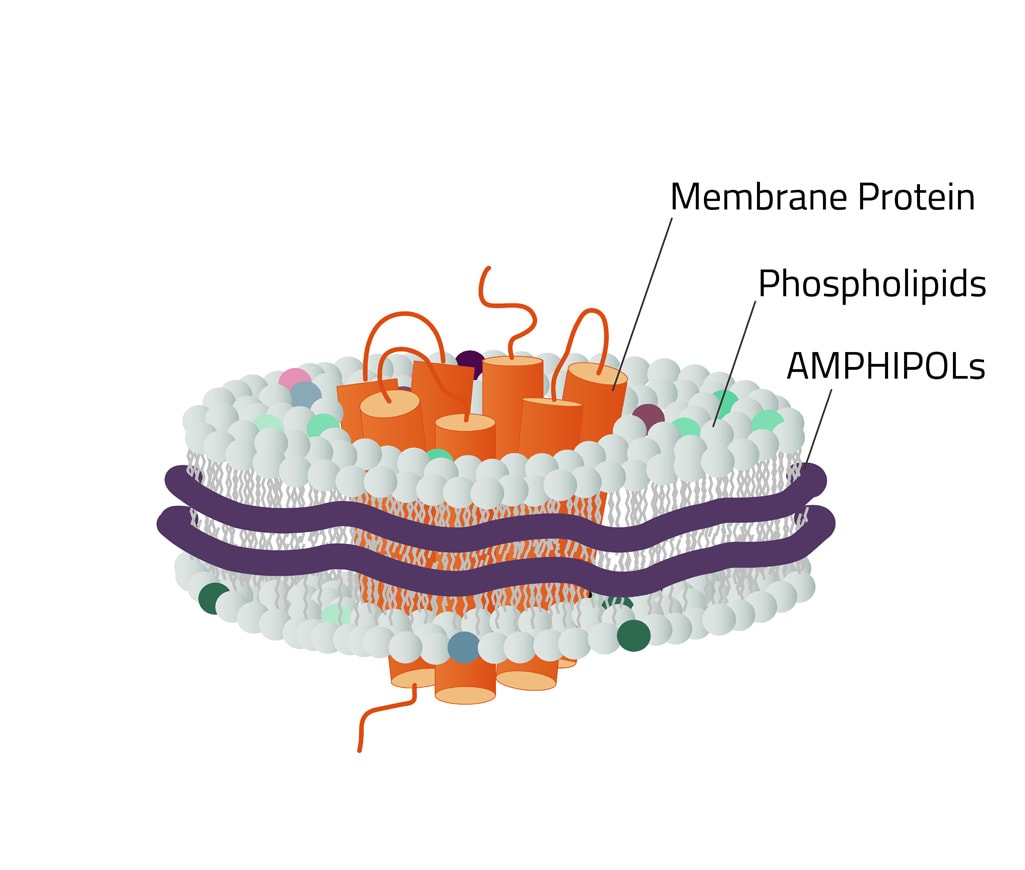
.png)
Copolymers provide a hydrophobic surface facing the lipids, and a hydrophilic surface on the outside. This setup makes nanodiscs highly soluble in aqueous solutions and allows for the solubilization of membrane proteins in the absence of detergents. The product can be used with phospholipids, such as dimyristoyl-glycero-phosphocholine (DMPC) or palmitoyl-oleoyl-phosphatidyl-choline (POPC) in combination with sodium cholate.
Possible Applications
The complex from UltrasoluteTM Amphipol and membrane protein can be used with many biophysical assays, such as SDS-PAGE, SEC, Western Blot, UV/Vis spectroscopy, and many chromatographic procedures.
| Name | Adsorbance (280 nm, 1% solution) | Molecular Weight | COA/AA Ratio | Solubility | pH (dissolved) | |
|---|---|---|---|---|---|---|
| Ultrasolute™ Amphipol 18 | > 0.1 | >7.300 g/mol | 50:50 | 10% (H2O) | 7.5 ± 0.3 | |
| Ultrasolute™ Amphipol 17 | > 0.1 | >7.300 g/mol | 50:50 | 10% (H2O) | 7.5 ± 0.3 |
What else is important to know?
As mentioned before, the history of amphipols can be dated back to Tribet in 1996. Back then, amphipols could only stabilize a membrane protein but they weren't able to solubilize it. This first task still needed to be done with detergents, similar to the handling of MSP nanodiscs. However, new developments make this handling obsolete. The new Ultrasolute™ Amphipols now can do both, as all of our nanodisc polymers can.
Amphipols combine two of the greatest advantages of other synthetic nanodisc polymers. First, their membrane protein solubilization efficiency is at least on par with SMA. Simultaneously, they also have no noteworthy absorption at wavelength 280nm, similar to DIBMA. Therefore, they won't interfere with protein quantification measurements in a photometer.
References
- Anaïs Marconnet, Baptiste Michon, Christel Le Bon, Fabrice Giusti, Christophe Tribet, and Manuela Zoonens ; Solubilization and stabilization of membrane proteins by cycloalkane-modified amphiphilic polymers, Biomacromolecules 2020, 21, 8, 3459–3467
- Anaïs Marconnet, Baptiste Michon, Bastien Prost, Audrey Solgadi, Christel Le Bon, Fabrice Giusti, Christophe Tribet, and Manuela Zoonens ; Influence of Hydrophobic Groups Attached to Amphipathic Polymers on the Solubilization of Membrane Proteins along with Their Lipids, Anal. Chem. 2022, 94, 41, 14151–14158
- Anna J. Higgins, Alex J. Flynn, Anaïs Marconnet, Laura J. Musgrove , Vincent L. G. Postis, Jonathan D. Lippiat , Chun-wa Chung, Tom Ceska, Manuela Zoonens, Frank Sobott, and Stephen P. Muench, Cycloalkane-modified amphiphilic polymers provide direct extraction of membrane proteins for CryoEM analysis, Communications Biology, volume 4, Article number: 1337, 2021
- Tribet, C., Audebert, R., Popot, JL. "Amphipols: Polymers that keep membrane proteins soluble in aqueous solutions". PNAS 93 (26), 15047-15050 (1996)





