GST-Tag - How A Protein Helps To Purify A Protein
Content:
1. What is the GST-tag?
2. What is GST / Glutathione-S-transferase?
3. Why GST from Schistosoma japonicum?
4. What are the GST-tag Features (Size, length, affinity, etc.)?
5. How to add/clone a GST-tag into a plasmid
6. How does GST-tag protein purification work?
7. How to remove a GST-tag?
What is the GST-tag?
There are multiple protein affinity tags that are being used for protein purification and the GST-tag is one of them. GST is short for Glutathione-S-transferase and refers to a whole protein, not just a few amino acids like the Rho1D4-tag. The GST enzyme tag has been in use for protein purification since the late 1980s (Smith & Johnson 1988) and has been established as a reliable way for protein purification assays that require the highest level of purity (Harper & Speicher 2013).
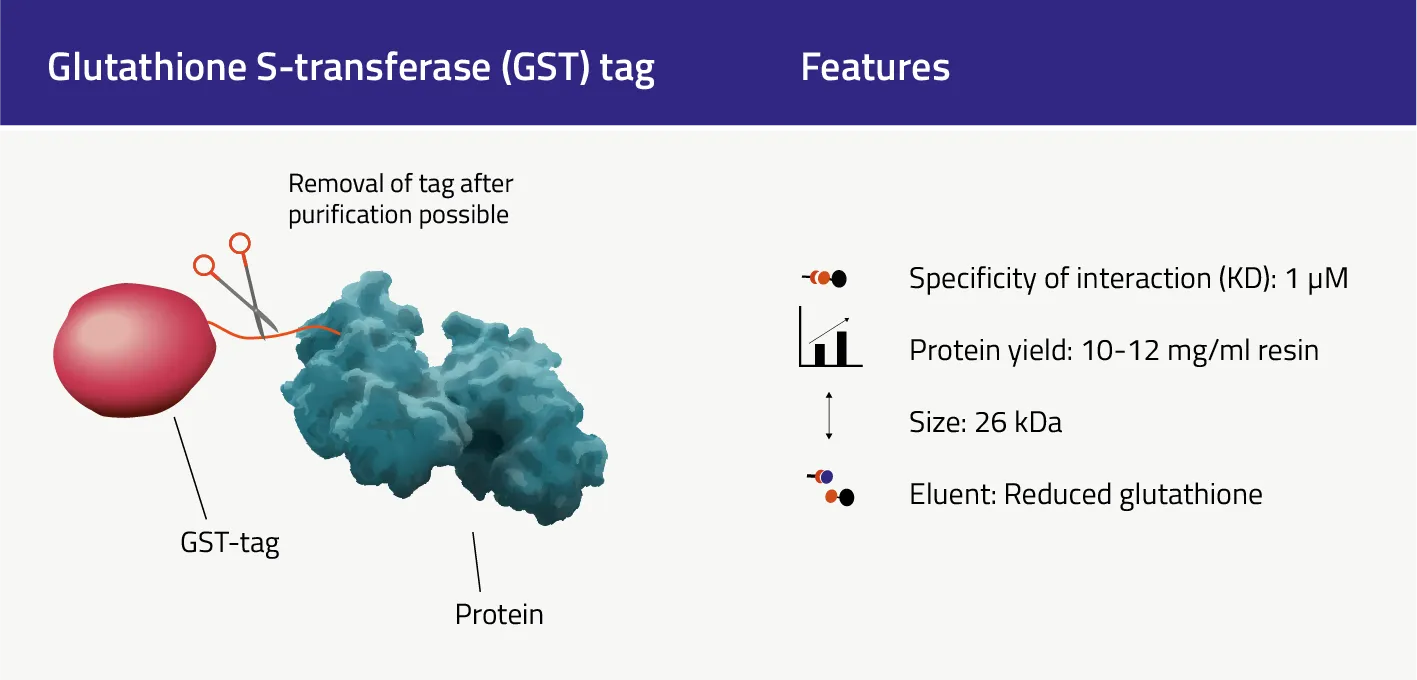
The GST-tag size is 26 kDa and therefore really large compared to other protein affinity tags. In theory, it can be fused to either the C- or N-terminus of a protein (Terpe 2003), but we highly recommend adding it to the N-terminus. So far it has been successfully used to purify proteins from bacterial cells (Smith and Johnson 1988), yeast (Lu et al. 1997), and mammalian cells (Rudert et al. 1996). Its general features & sequence can be found here.
So we know now, that the GST-tag basically is a whole protein that gets attached to the protein of interest. But what is the Glutathione-S-transferase now exactly?
The GST-tag size is 26 kDa and therefore really large compared to other protein affinity tags. In theory, it can be fused to either the C- or N-terminus of a protein (Terpe 2003), but we highly recommend adding it to the N-terminus. So far it has been successfully used to purify proteins from bacterial cells (Smith and Johnson 1988), yeast (Lu et al. 1997), and mammalian cells (Rudert et al. 1996). Its general features & sequence can be found here.
So we know now, that the GST-tag basically is a whole protein that gets attached to the protein of interest. But what is the Glutathione-S-transferase now exactly?
What is GST / Glutathione-S-Transferase?
Glutathione-S-transferases are a family of prokaryotic and eukaryotic enzymes that are mainly involved in the detoxification of the cells and body. In the most extreme cases, GST proteins can make up to 10 % of the whole cytosolic proteome of some mammalian cells (Oakley 2011). The reaction Glutathione S-Transferases are catalyzing is the oxidation of reduced Glutathione, also called GSH. GST's main function in the cell is the protection of DNA and cellular proteins from external toxic substances. The mechanism catalyzes a nucleophilic attack on the toxic substance. By activating a thiol group of GSH, it reacts with suitable groups on the toxic substances. This coupling of GSH to the toxins leads to their excretion from the cell or further metabolic procession (Hayes et al. 2004). The high specificity of GST towards reduced Glutathione (GSH) is the reason for the high protein purity of the purification assays.
_Complete process.webp)
As stated: There are numerous GST orthologs out there, but a specific one was chosen to be the template for the GST-tag. So why GST from Schistosoma japonicum?
Why GST from Schistosoma Japonicum?
Glutathione-S-transferases can be found in organisms ranging from prokaryotes to eukaryotes (Allocati et al. 2008). So why is this specific GST protein used for affinity purification? What are its advantages? This answer derives back to the first description of GST as an option for affinity chromatography and protein purification. As formally cited, the first mention of GST for protein purification was in 1988 by Smith & Johnson. Before that Smith et al. published a paper in which they described the possible use of GST as a vaccine against Schistosoma japonicum.
Schistosoma japonicum, a Platyhelminthes is a major cause of the disease schistosomiasis and the GST protein of said species showed promise to be the base for a vaccine against Schistosoma japonicum (Smith et al. 1988). Because of this study, Smith already worked with this specific ortholog of the GST protein, and therefore it remained the base for the later described protein purification method. And since 1988 GST from Schistosoma japonicum, remained as the GST ortholog that is used as the GST affinity tag.
GST-tag Features
We compiled the most important data regarding the GST-tag and its capabilities for protein purification.
Note: We listed the "standard" sequence used in pGEX vectors.
Table 1: Overview over the most important features of the GST-tag
Note: We listed the "standard" sequence used in pGEX vectors.
Table 1: Overview over the most important features of the GST-tag
| Features of the GST-tag: | |
|---|---|
| Amino acid Sequence | MSPILGYWKIKGLVQPTRLLLEYLEEKYEE HLYERDEGDKWRNKKFELGLEFPNLPYYID GDVKLTQSMAIIRYIADKHNMLGGCPKERA EISMLEGAVLDIRYGVSRIAYSKDFETLKV DFLSKLPEMLKMFEDRLCHKTYLNGDHVTH PDFMLYDALDVVLYMDPMCLDAFPKLVCFK KRIEAIPQIDKYLKSSKYIAWPLQGWQATF GGGDHPPKSD |
| DNA sequence (Based on pGEX-4T1 vector) |
ATG TCCCCTATAC TAGGTTATTG GAAAATTAAG GGCCTTGTGC AACCCACTCG ACTTCTTTTG GAATATCTTG AAGAAAAATA TGAAGAGCAT TTGTATGAGC GCGATGAAGG TGATAAATGG CGAAACAAAA AGTTTGAATT GGGTTTGGAG TTTCCCAATC TTCCTTATTA TATTGATGGT GATGTTAAAT TAACACAGTC TATGGCCATC ATACGTTATA TAGCTGACAA GCACAACATG TTGGGTGGTT GTCCAAAAGA GCGTGCAGAG ATTTCAATGC TTGAAGGAGC GGTTTTGGAT ATTAGATACG GTGTTTCGAG AATTGCATAT AGTAAAGACT TTGAAACTCT CAAAGTTGAT TTTCTTAGCA AGCTACCTGA AATGCTGAAA ATGTTCGAAG ATCGTTTATG TCATAAAACA TATTTAAATG GTGATCATGT AACCCATCCT GACTTCATGT TGTATGACGC TCTTGATGTT GTTTTATACA TGGACCCAAT GTGCCTGGAT GCGTTCCCAA AATTAGTTTG TTTTAAAAAA CGTATTGAAG CTATCCCACA AATTGATAAG TACTTGAAAT CCAGCAAGTA TATAGCATGG CCTTTGCAGG GCTGGCAAGC CACGTTTGGT GGTGGCGACC ATCCTCCAAA ATCGGATCTG GTTCCGCGTG GATCCCCGGA ATTCCCGGGT CGACTCGAGC GGCCGCATCG TGACTGA |
| Size | 26 kDa |
| Compatibility to recombinant proteins | N-terminus is highly recommended |
| Combinable with other affinity tags? | Yes, see our GST-and-Rho-tagged eGFP as an example |
| Specificity of interaction (KD) | 1 µM |
| Typical protein yield per ml of high-quality purification resin. | 10-12 mg/ml |
| Elution conditions | Reduced Glutathione, pH shift, or tag cleavage |
| Binds to purification resin / magnetic bead via | Reduced Glutathione |
| Suitable protein purification methods | Agarose resin & magnetic beads |
How to add/clone a GST-tag into a Plasmid
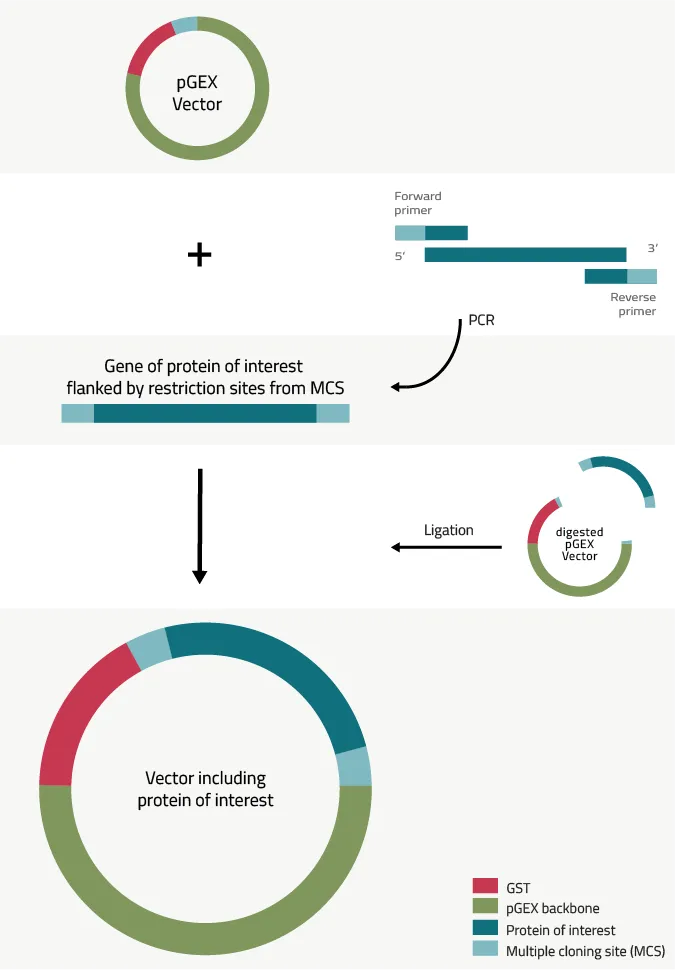
The best way to add a GST-tag to a protein of interest is using a vector of the pGEX series. They also come with different protein cleavage sites. These are important for the later mentioned methods to remove a GST-tag. Figure 4 depicts the process and steps of our recommended cloning strategy.
1. The pGEX vectors all come with a multiple cloning site (MSC) that contains multiple restriction enzyme digestion sites. These vary from pGEX vector to vector. Table 2 provides an overview here. In addition, all pGEX vectors contain three stop codons that cover all three possible reading frames on a DNA strain, after the MSC. This is to ensure that the expressed protein does not have to contain the stop codon by itself.
2. The next step is to create a PCR product of your gene/protein of interest that is flanked in both directions with restriction sites that are present in the MCS of the chosen pGEX vector. These primers should cover each:
Let us give you an example of this: Let us try to assume that you want to add a GST-tag to eGFP.
1. The pGEX vectors all come with a multiple cloning site (MSC) that contains multiple restriction enzyme digestion sites. These vary from pGEX vector to vector. Table 2 provides an overview here. In addition, all pGEX vectors contain three stop codons that cover all three possible reading frames on a DNA strain, after the MSC. This is to ensure that the expressed protein does not have to contain the stop codon by itself.
2. The next step is to create a PCR product of your gene/protein of interest that is flanked in both directions with restriction sites that are present in the MCS of the chosen pGEX vector. These primers should cover each:
- 3-4 nts overhang (the longer, the better for restriction digest, but we suggest 3-4)
- The restriction site
- 20-25 nts of your gene of interest
Let us give you an example of this: Let us try to assume that you want to add a GST-tag to eGFP.
Legend - Forward primer:
Overhang
Restriction site -> BamHI in this case
First 20 nts of eGFP
5'-GGG GGATCC ATGAAACATCACCATCACCA-3'
Overhang
Restriction site -> BamHI in this case
First 20 nts of eGFP
5'-GGG GGATCC ATGAAACATCACCATCACCA-3'
Legend - reverse Primer:
Overhang
Restriction site -> EcoRI in this case, reverse complement
The last 20 nts of eGFP, reverse complement
5'-GGG GGATTC TTTGTATAGTTCATCCATGC-3'
Overhang
Restriction site -> EcoRI in this case, reverse complement
The last 20 nts of eGFP, reverse complement
5'-GGG GGATTC TTTGTATAGTTCATCCATGC-3'
Please note that this presented strategy is only one example of how a GST-tag can be added to a protein of interest. Other methods like homologous recombination in yeast or bacteria are also possible ways to accomplish this.
Table 2: Different pGEX vectors and the restriction sites inside their MCSs, sorted in 5' to 3' direction.
| pGEX vector | Restriction sites in 5' to 3' direction |
|---|---|
| pGEX-1λT | BamHI , EcoRI |
| pGEX-2T | BamHI, SmaI, EcoRI |
| pGEX-2TK | BamHI, SmaI, EcoRI |
| pGEX-4T-1 | BamHI, EcoRI, SmaI, SalI, XhoI, NotI |
| pGEX-4T-2 | BamHI, EcoRI, SmaI, SalI, XhoI, NotI |
| pGEX-4T-3 | BamHI, EcoRI, SmaI, SalI, XhoI, NotI |
| pGEX-3X | BamHI, SmaI, EcoRI, |
| pGEX-5X-1 | BamHI, EcoRI, SmaI, SalI, XhoI, NotI |
| pGEX-5X-2 | BamHI, EcoRI, SmaI, SalI, XhoI, NotI |
| pGEX-5X-3 | BamHI, EcoRI, SmaI, SalI, XhoI, NotI |
| pGEX-6P-1 | BamHI, EcoRI, SmaI SalI, XhoI, NotI |
| pGEX-6P-2 | BamHI, EcoRI, SmaI SalI, XhoI, NotI |
| pGEX-6P-3 | BamHI, EcoRI, SmaI SalI, XhoI, NotI |
How does GST-tag Protein Purification work?
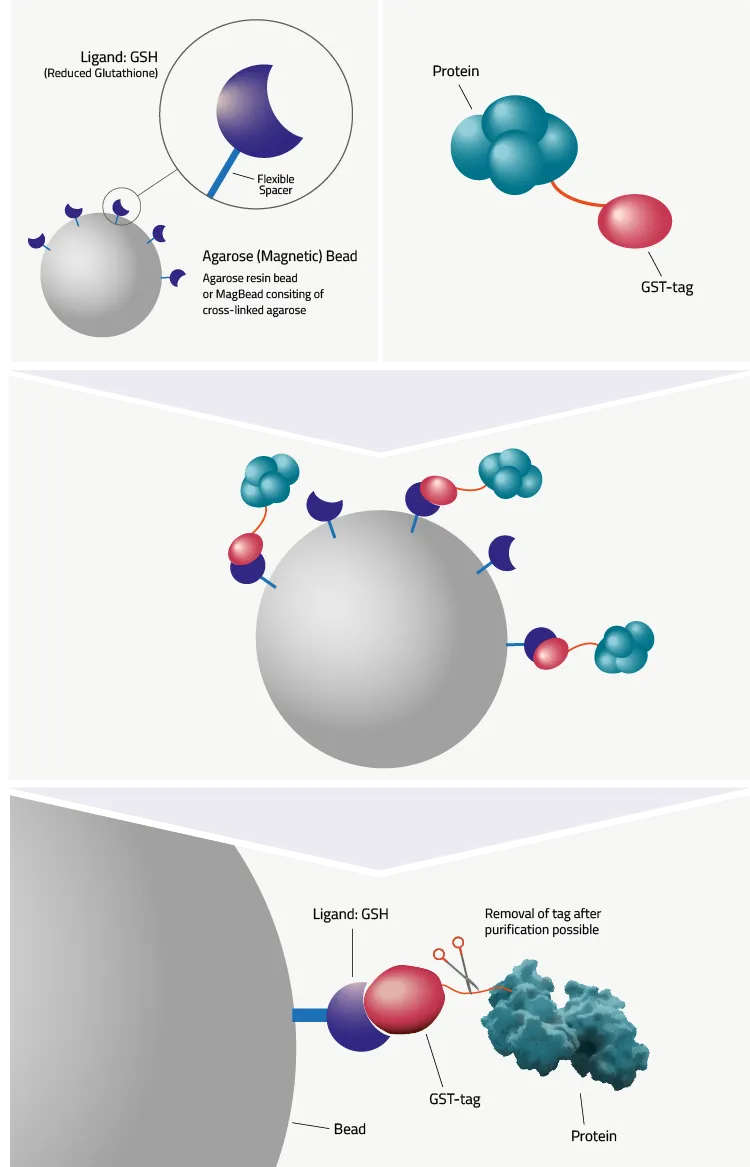
GST-tag protein purification is an affinity chromatography method. It exploits the high affinity of GST towards reduced Glutathione, its natural substrate. To achieve this Glutathione is bound to either agarose resin or magnetic beads, depending on the preferred purification method. Figure 5 shows a schematic depiction of such a bead.
The actual purification protocol differs from supplier to supplier of GST-tag affinity products. These differences can also include different buffer compositions that are only optimized for their own specific GST affinity beads. Therefore we highly recommend not applying protocols for GST-tag protein purification from one supplier to another supplier's product.
You can find Cube Biotech's GST-tag affinity products here.
The corresponding protocols are listed here:
Agarose Resin GST-tag purification protocol:
Magnetic Bead GST-tag purification protocol:
The corresponding protocols are listed here:
Agarose Resin GST-tag purification protocol:
Magnetic Bead GST-tag purification protocol:
GST-tag Protein
Purification Protocol
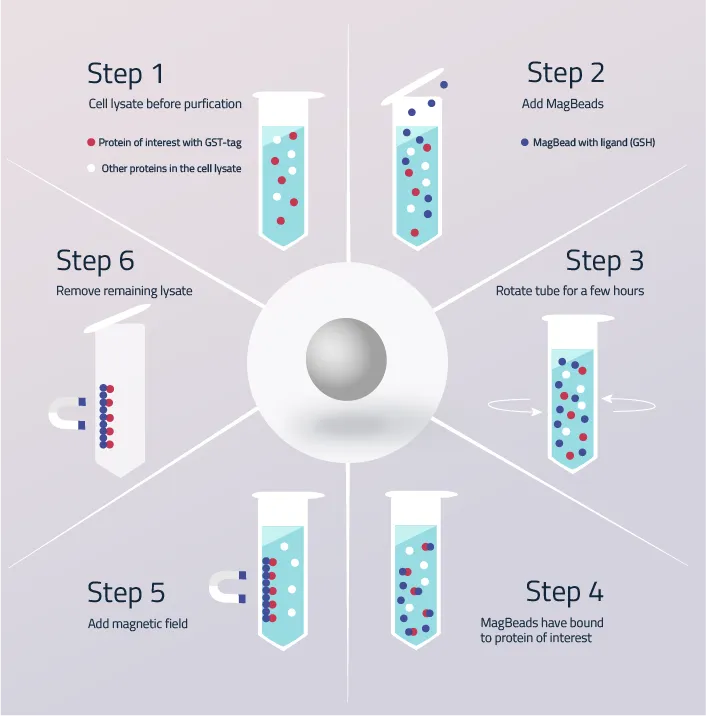
The protein purification with GST affinity beads is presented in figure 5. These core steps are based on the purification procedure using GST-tag affinity magnetic beads.
Step 1: The cell lysate after cell lysis. The whole proteome of the cell floats inside the lysis buffer. GST-tagged proteins are unseparated from the other proteins.
Step 2: Addition of GST-tag Affinity MagBeads to the cell lysate. More MagBeads should be added than GST-tagged protein is suspected inside the cell lysate.
Step 3: Rotation of the cube/flask for a couple of hours to give the glutathione beads enough time to bind all GST-tagged proteins inside the cell lysate.
Step 4: GST-tagged proteins have now bound to the MagBeads. Due to the specificity of the GST-tag to the Glutathione on the beads, no other proteins should have bound.
Step 5: Application of a magnetic force onto the cell lysate. The GST-tagged proteins have now bound to the Glutathione beads, while no other protein has.
Step 6: Removal of the unbound cell lysate/protein. The GST-tagged proteins are now purified.
To verify the success of a GST-tag purification, we recommend performing a Western Blot that uses Anti-GST antibodies to detect the purified protein. A Western Blot example protocol can be found here.
How to remove a GST-tag?
With 26 kilodaltons (kDa) the GST-tag is one of the largest affinity tags out there. As it is in fact a whole protein, it can even surpass some proteins that it gets attached to. So of course this creates the valid concern that an artificial attachment of this size to a protein can interfere with said protein's functionality. Therefore the question arises if it is possible to remove a GST-tag after protein purification to ensure that it does not hinder the purified protein in any way.
This concern was already in the mind of the creators of the pGEX vector series, which we already mentioned in table 2. By creating a GST-tag fusion protein in the way described above in the chapter "How to add/clone a GST-tag into a plasmid" you get the option to remove this GST-tag later on.
The answer is to add a specific protein cleavage site in 5' direction of the MCS of the plasmid. This way the N-terminal GST-tag can be cleaved off, even after purification. This can also be used as an elution method during purification instead of the elution buffer that is mentioned in our agarose resin and magnetic bead purification protocols.
Since this method includes a small chance that your protein of interest includes a protease cleavage site by coincidence there are three different options of protein cleavage sites that are already present in the pGEX vectors.
This concern was already in the mind of the creators of the pGEX vector series, which we already mentioned in table 2. By creating a GST-tag fusion protein in the way described above in the chapter "How to add/clone a GST-tag into a plasmid" you get the option to remove this GST-tag later on.
The answer is to add a specific protein cleavage site in 5' direction of the MCS of the plasmid. This way the N-terminal GST-tag can be cleaved off, even after purification. This can also be used as an elution method during purification instead of the elution buffer that is mentioned in our agarose resin and magnetic bead purification protocols.
Since this method includes a small chance that your protein of interest includes a protease cleavage site by coincidence there are three different options of protein cleavage sites that are already present in the pGEX vectors.
Table 3: Different pGEX vectors and their included protease cleavage site.
| pGEX vector | Protease Cleavage site in 5' of the MCS |
|---|---|
| pGEX-1λT; pGEX-2T; pGEX-2TK; pGEX-4T-1; pGEX-4T-2; pGEX-4T-3 | Thrombin |
| pGEX-3X; pGEX-5X-1; pGEX-5X-2; pGEX-5X-3 | Factor Xa |
| pGEX-6P-1; pGEX-6P-2; pGEX-6P-3 | PreScission Protease |
GST-tag removal protocol (example)
Note: This protocol describes how to cleave off a GST-tag using the thrombin cleavage site from free eluted GST fusion proteins.
Table 4: Required reagents for Thrombin cleavage
| Reagent | Amount |
|---|---|
| Thrombin | Depends on the supplier: 3-10 U of thrombin can cleave 1 mg of fusion protein at RT overnight on average. Still, read the supplier's notes. |
| 1 X PBS buffer | 140mM NaCl; 2.7mM KCl; 10mM Na2HPO4; 1.8mM KH2PO4; pH 7.3 |
| PMSF | Enough for a 1 mM concentration in your protein solution |
| AEBSF | Alternative to PMFS; Enough for a 1mM concentration in your protein solution |
Procedure (general version)
- Add to the eluate or column purification 10 cleavage units of thrombin ~10 µl per milligram of GST fusion protein
- Mix gently and incubate the mixture at room temperature (22°C) for 2-16 hours.
- After the digest, you can stop the reaction by either adding 1 mM of PMSF or ABESF
- Check the results via SDS page OR
- Directly separate the cleavage products by SDS chromatography (e.g. Size exclusion chromatography)
Tip: You can incubate a sufficient amount of your GST-tagged protein to see on SDS-Page gels with 0.01 to 0.06 units of thrombin and different digestion times (e.g. 2h, 4h, 6h, etc.). Stop the reaction as described and store the aliquots at -20°C until use.
References
- Hayes, J. D., Flanagan, J. U. & Jowsey, I. R. GLUTATHIONE TRANSFERASES. Annu. Rev. Pharmacol. Toxicol. 45, 51–88 (2004).
- Allocati, N., Federici, L., Masulli, M. & Di Ilio, C. Glutathione transferases in bacteria. The FEBS Journal 276, 58–75 (2009).
- Oakley, A. Glutathione transferases: A structural perspective. Drug metabolism reviews 43, 138–51 (2011).
- Smith, D. B. et al. Mr 26,000 antigen of Schistosoma japonicum recognized by resistant WEHI 129/J mice is a parasite glutathione S-transferase. Proc Natl Acad Sci U S A 83, 8703–8707 (1986).
- Terpe, K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Applied Microbiology and Biotechnology 60, 523–533 (2003).
- Rudert, F. et al. pLEF, a novel vector for expression of glutathione S-transferase fusion proteins in mammalian cells. Gene 169, 281–282 (1996).
- Harper, S. & Speicher, D. W. Purification of proteins fused to glutathione S-transferase. Methods Mol Biol 681, 259–280 (2011).
- Smith, D. B. & Johnson, K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67, 31–40 (1988).
- Lu, Q., Bauer, J. C. & Greener, A. Using Schizosaccharomyces pombe as a host for expression and purification of eukaryotic proteins. Gene 200, 135–144 (1997).


