Guide to magnetic beads for protein purification
Content:
The workflow with Magnetic Beads is surprisingly straightforward. To achieve protein purity, simply follow the six easy-to-perform steps that are depicted in figure 1.
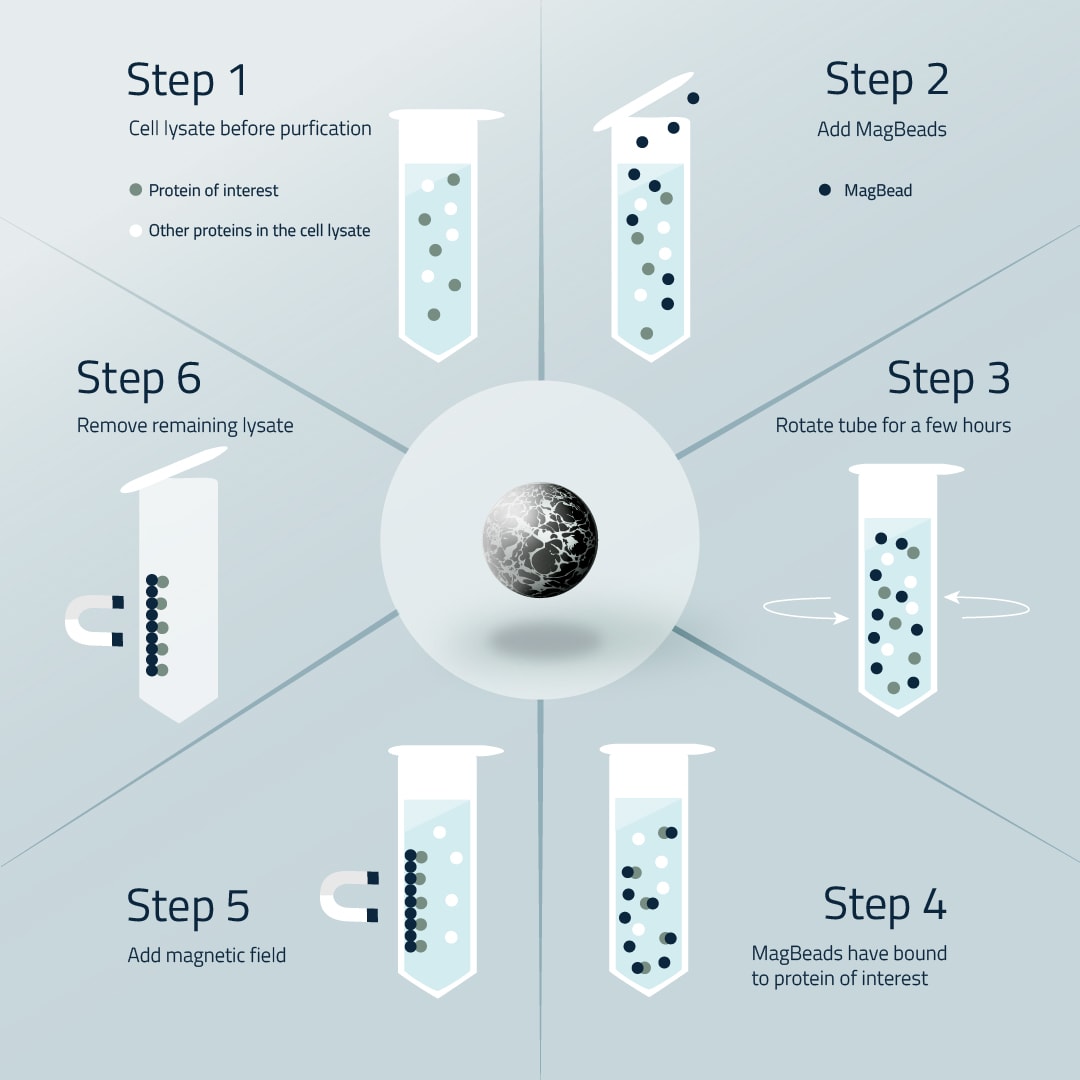
Workflow of MagBead protein purification step-by-step in a listed guide
-
Step 1:The usual starting point of MagBead protein purification is a cell lysate in which the proteins of interest are mixed together with every other protein the expression cells had produced simultaneously.
-
Step 2:Remove the storage buffer from the magnetic beads and wash two times with wash buffer. Add now an appropriate amount of magnetic beads to the lysate. We suggest that you use sterile pipette tips for this.
-
Step 3:Shake the MagBead/Protein mixture. We suggest automated mixing with a slower but steady speed. No vortexing! A duration of around 2 hours at room temperature is what we see as sufficient. Figure 1 may only show a microcentrifuge tube but a sterile falcon tube is also as well suited.
-
Step 4:Now, almost every protein of interest should have bound to a MagBead. Again, it is important to use more magnetic beads than protein of interest is present to ensure maximum binding effectiveness.
-
Step 5:Apply a magnetic force to your magnetic beads to separate them and in turn the protein of interest from the rest of the lysate. For the microcentrifuge tube, we suggest our practical MagBead separator for this. Or our CubeMax MagBead separator for large scale purifications.
-
Step 6:In the final step, the rest of the cell lysate that did not bind to the magnetic beads will be removed using again sterile peptide tips. Keep the magnetic force active to avoid accidental removal of some magnetic beads. Now wash the bound proteins at least two times with washing buffer.
Elute your proteins with elutions buffer. Vortex your beads slowly & steadily with elution buffer to elute all bound proteins. -
Optional:Additional washing steps will increase the purity of your protein. Different types of MagBeads can require different kinds of washing buffers. To see how the corresponding washing buffer for your MagBeads is prepared, consult our protocols & datasheets page.You will also find the necessary information to elute the proteins of interest from the magnetic beads later on if this is necessary for you. Feel free to contact us in case you have any further questions.
Watch our comprehensive guide to magnetic beads by Roland Fabis, Ph.D.:
The advantages of magnetic beads / MagBeads in detail
Using magnetic beads for protein purification has a number of advantages compared to non-magnetic affinity matrices. At the same time, there are a lot of different products available with different features and benefits. This short summary shall support you in finding the optimal product for your research.

How can magnetic beads purify proteins?
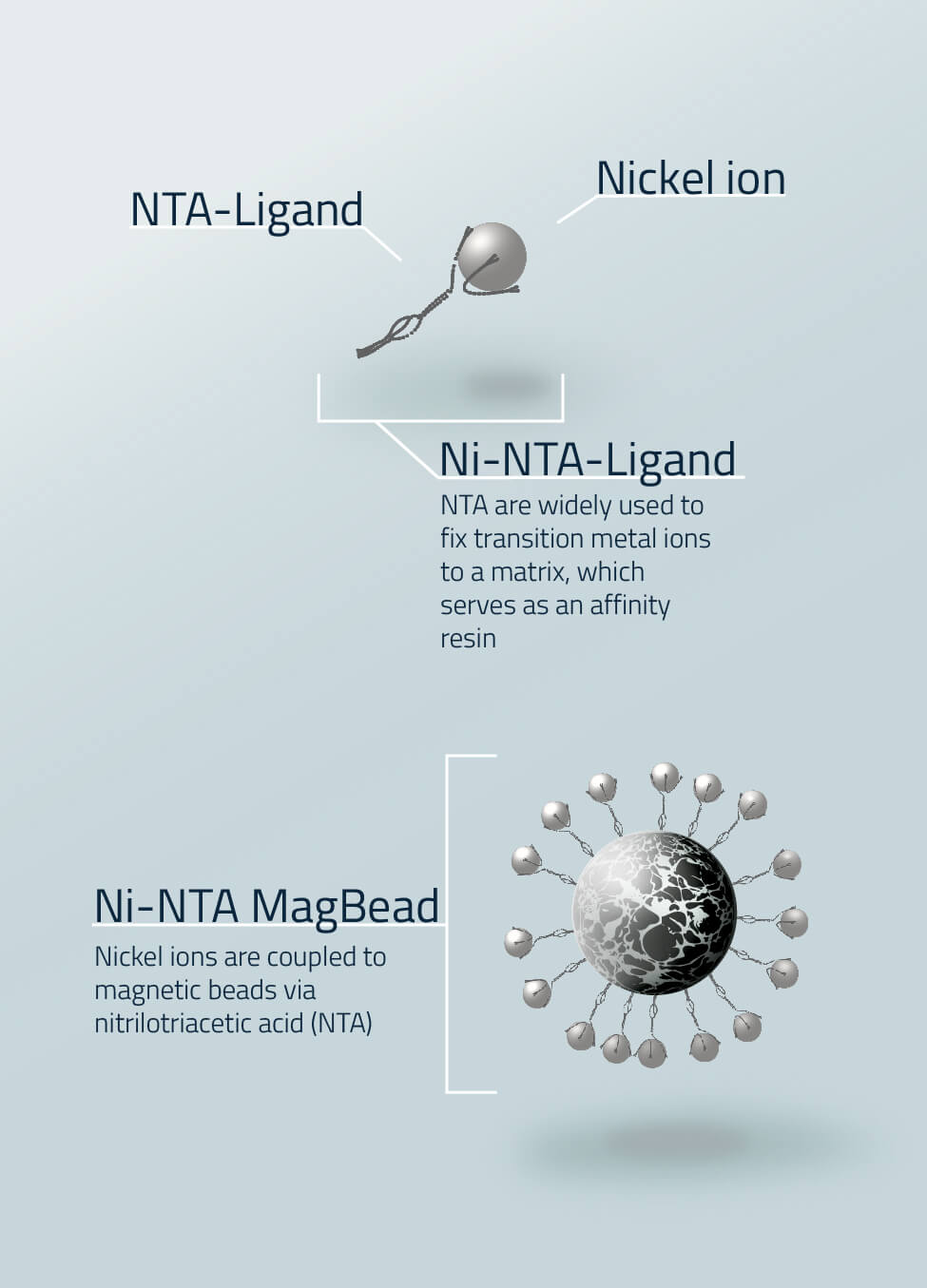
The magnetic bead itself cannot interact with a protein. It has to be loaded with ions. Different metal ions result in differing affinity and specificity for a his-tagged protein . The most used tool to purify proteins via affinity chromatography is the nickel-NTA ligand (Fig. 2). The nitrilotriacetic acid (NTA) ligand is coupled to matrices like magnetic beads and then loaded with ions. The ions interact with specific tagged proteins (e.g. 6x his tagged proteins). In figure 4 nickel ions are loaded to a magnetic bead. Although the nickel ions are light blue the magnetic beads appear black because of the magnetic cores.
Magnetic Core
Magnetic beads used in protein purification contain a magnetic core made of magnetite which is covered in different materials. Magnetic beads are typically either ferri (or ferro-) magnetic, or superparamagnetic.
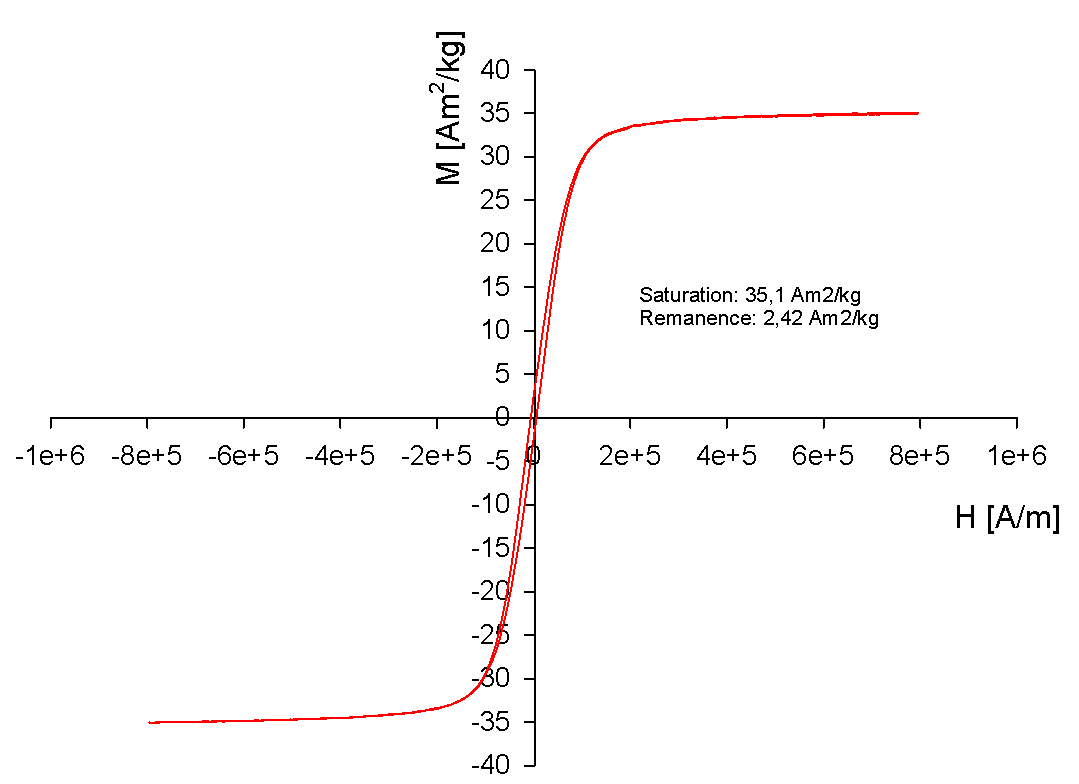
- Ferri/Ferromagnetic cores are typically large (>30 nm) and show a strong magnetic moment. They retain this magnetic moment even after the removal of the magnetic field. This effect is called "magnetic remanence". The strong magnetic field leads to a fast separation of the beads in the magnetic field. At the same time, they sometimes show self-magnetism and may attach to metal surfaces. PureCube magnetic beads are by default ferrimagnetic.
- Superparamagnetic magnetic cores are smaller (5-30 nm), and their magnetic moment is weaker. When the outer magnetic field is removed, the beads lose their magnetism. Separation of the beads in the magnetic field typically takes longer or is less efficient. At the same time, use with metal surfaces is facilitated. In case you have a need for superparamagnetic magnetic beads feel free to contact us.
PureCube MagBead Properties
For our standard magnetic beads, we chose the following properties to provide optimal results for most applications. However, we can provide a range of customized magnetic beads if required.
- Standard: Medium-sized beads (20-40 µm) with a ferrimagnetic core, and agarose coating: For high protein binding, low unspecific binding, and efficient separation
- XL: Large or extra-large magnetic agarose beads (70-120 µm or up to 400 µm) for special applications are available, and also other commercially available magnetic supports can be modified with your affinity ligand of choice. Contact us to learn more.
Customized magnetic beads
If your needs for magnetic beads exceed the common repertoire of the market you can contact us and describe what specific features your magnetic beads need for your experiments. We have already fulfilled a magnitude of customized orders regarding magnetic beads. Check this overview to see what is possible.
For example, the following features might be of interest for you to customize:
Surface Chemistry
The magnetic core can be covered in a range of different materials, providing different properties.
- Agarose forms a three-dimensional hydrophilic mesh with a neutral charge. Similar to non-magnetic agarose, it is very well suited to bind to proteins, e.g. via affinity ligands. The large interacting surface leads to high binding capacities. The neutral surface reduces non-specific binding.
- Polyvinyl beads have a carboxylated surface which can be rather rough (by addition of carboxylate brushes), or rather smooth. Both variants sometimes show unspecific binding to proteins. Their surface area available for protein binding is smaller than that of agarose-coated beads, therefore they are typically smaller in size (1-2 µm) to provide the same binding capacity.
- Silica beads are most widely used for nucleic acid purification. They become negatively charged at pH, and often show unspecific binding in protein purification.
Size
Magnetic beads come in different sizes, which has an impact on handling and purification results.
- Small beads (1-10 µm): provide high surface areas available for protein purification, but a smaller magnetic field, which can negatively influence separation, especially from viscous solutions.
- Medium beads (20-40 µm): provide efficient separation and large surface area. When combined with agarose surfaces, binding capacities can be in the same range as for small polyvinyl beads.
- Large beads (70-120 µm): have advantages for special applications, e.g. when purification methods contain both magnetic separation and filtration. When combined with agarose surfaces, binding capacities are still high.
Magnetic Bead Products
Cube Biotech offers many different magnetic bead products. We want to give you a short overview: Your product is not listed? No problem, we can manufacture it for you!



