INDIGO: Common problems of His-tag purification solved!
The work with eukaryotic cell lines like HEK293 provides the best insights on human proteins. However working with them requires more attention and care compared to other cell cultures like E.coli cell cultures. This also applies to protein purification assays.
One major problem that can decrease protein yields are proteases from the eukaryotic cells themselves (Scopes 1994). To prevent their unwanted activity during native cell lysis, protease inhibitor cocktails are often added to the lysis buffers.
Popular components of these protease inhibitor cocktails are Phenanthroline and/or EDTA/EGTA as chelators to inhibit metalloproteases. These substances are strong chelators that bind divalent cations, which are essential co-factors for cellular metalloproteases. Thus, their removal makes the proteases inactive (Day & Chen 1998; Huyer et al. 1997).
However, these chelating substances do not stop at cellular cations. They also tend to bind the cations bound to His-tag affinity beads. E.g. Nickel ions on Ni-NTA based beads. In other words they make His-tag affinity beads useless (Crowe et al. 1994).
This would usually lead to the following dilemma: Either one does not use protein inhibitor cocktails and risks lower protein yields. Or one uses them and risks damaging a part of the His-affinity beads, resulting in an equally as noticeable decrease in protein yield.
But the emphasis in this sentence lies on the word "usually". Because there is a solution to this problem and it is called INDIGO!
One major problem that can decrease protein yields are proteases from the eukaryotic cells themselves (Scopes 1994). To prevent their unwanted activity during native cell lysis, protease inhibitor cocktails are often added to the lysis buffers.
Popular components of these protease inhibitor cocktails are Phenanthroline and/or EDTA/EGTA as chelators to inhibit metalloproteases. These substances are strong chelators that bind divalent cations, which are essential co-factors for cellular metalloproteases. Thus, their removal makes the proteases inactive (Day & Chen 1998; Huyer et al. 1997).
However, these chelating substances do not stop at cellular cations. They also tend to bind the cations bound to His-tag affinity beads. E.g. Nickel ions on Ni-NTA based beads. In other words they make His-tag affinity beads useless (Crowe et al. 1994).
This would usually lead to the following dilemma: Either one does not use protein inhibitor cocktails and risks lower protein yields. Or one uses them and risks damaging a part of the His-affinity beads, resulting in an equally as noticeable decrease in protein yield.
But the emphasis in this sentence lies on the word "usually". Because there is a solution to this problem and it is called INDIGO!
Cube Biotech presents its in-house developed Ni-INDIGO resin:
His-tag based protein purification assays are usually performed with agarose or magnetic beads coupled to ligand-ion complexes like Ni-NTA and Ni-IDA.
These beads are a staple in biotechnology, we have them in our His-tag products store. Cube Biotech has developed a new generation of products for His-tag protein purification with impressive features.
These beads are a staple in biotechnology, we have them in our His-tag products store. Cube Biotech has developed a new generation of products for His-tag protein purification with impressive features.
What are INDIGO's key features?
INDIGO was developed to solve two problems occurring during His-tag protein purifications:
- The tolerance to certain cell buffer ingredients
- The comparatively low purity of His-tag purifications in general
The main idea was to keep the standard purification protocol that was used with e.g. Ni-NTA beads unchanged. Everything remains unchanged, except the usage of agarose beads / magnetic beads.
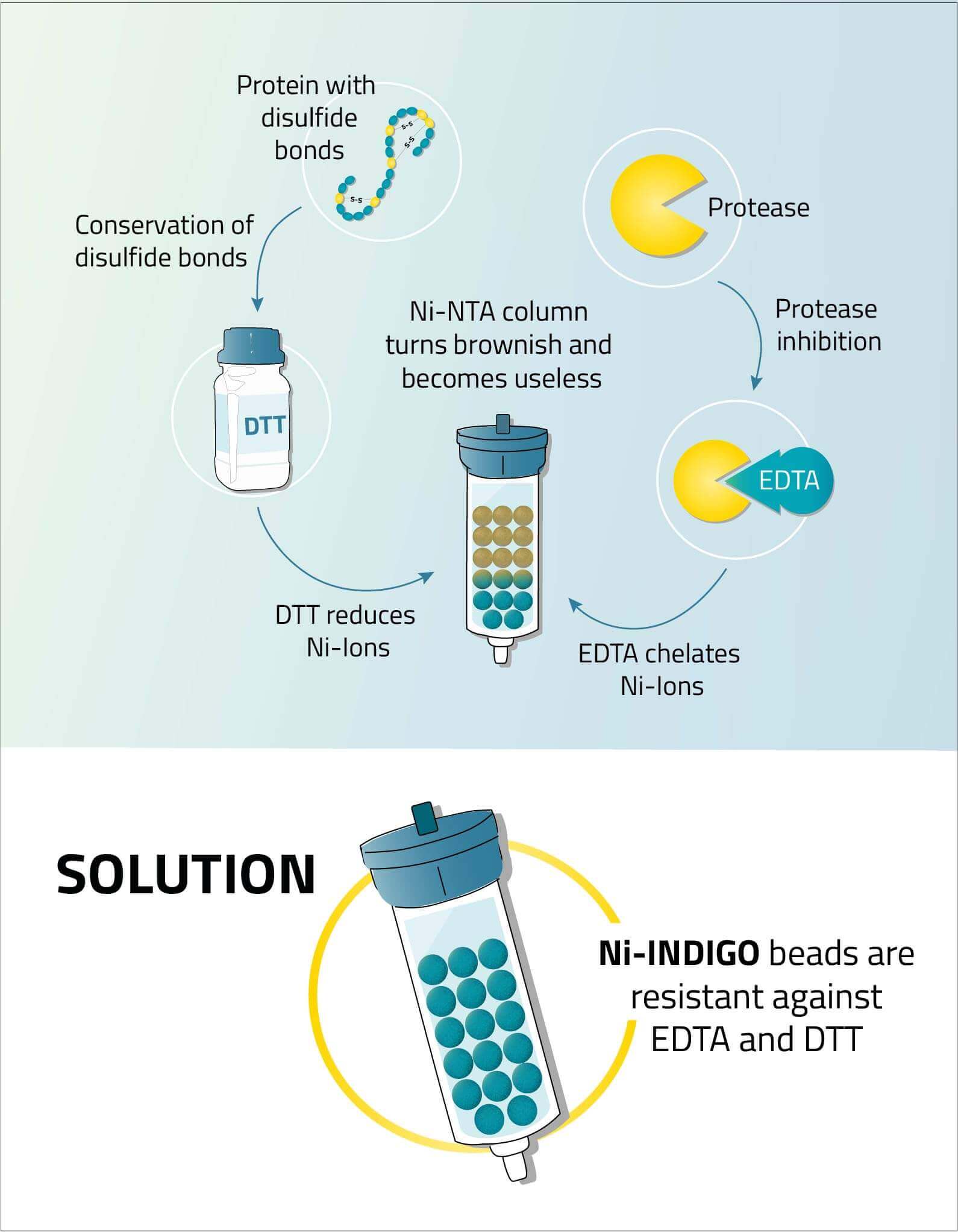
The chelator ligand is mostly responsible for the tolerances against chemicals, capacity and purity. Therefore the goal was to develop and introduce a ligand that withstands commonly used reagents like EDTA, DTT and phenanthroline to give the user a huge benefit.
The chelator ligand is mostly responsible for the tolerances against chemicals, capacity and purity. Therefore the goal was to develop and introduce a ligand that withstands commonly used reagents like EDTA, DTT and phenanthroline to give the user a huge benefit.
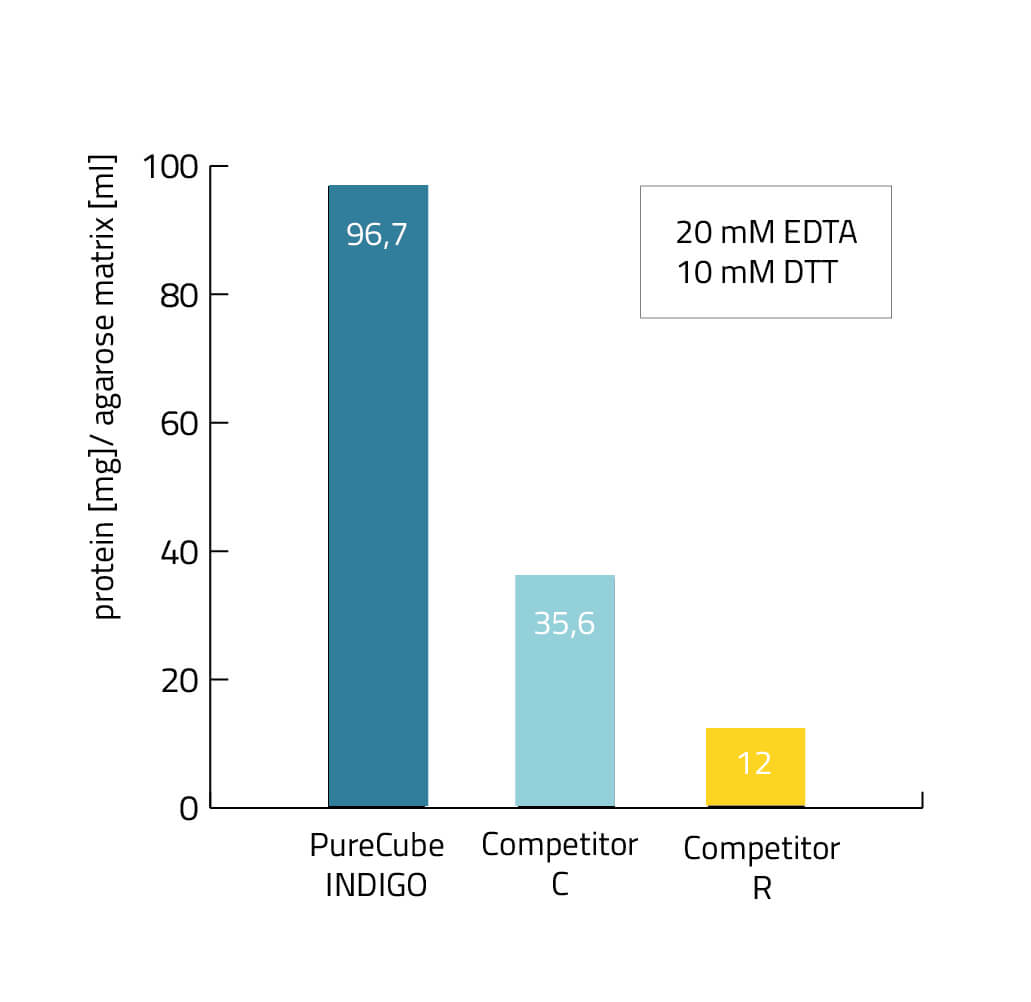
EDTA and DTT resistance of the INDIGO ligand
Protein purification is not limited to simple organisms like E.coli. More complex cells like mammalian cells or insect cell lines can also be used to express and purify proteins. These systems come with more complex buffer compositions and some of their ingredients may interfere with traditional IMAC ligands. These include:
EDTA is often used in mammalian cell buffers to inhibit any proteases that decrease the protein yield. However, it also strips the NTA and IDA ligands of their nickel ions, thus making the beads useless.
DTT on the other hand can be used to dissolve protein aggregates in the cell lysate that might hinder the access to the protein's His-tag. However, it reduces the nickel cations on the beads, thus making the beads useless.
Phenanthroline as a protease inhibitor is a strong chelator. And therefore behaves similar to EDTA.
To address all these issues, our research and development team developed our INDIGO ligand, which has an increased tolerance to EDTA, DTT and phenanthroline during a His-tag purification procedure up to 20 mM each.
EDTA is often used in mammalian cell buffers to inhibit any proteases that decrease the protein yield. However, it also strips the NTA and IDA ligands of their nickel ions, thus making the beads useless.
DTT on the other hand can be used to dissolve protein aggregates in the cell lysate that might hinder the access to the protein's His-tag. However, it reduces the nickel cations on the beads, thus making the beads useless.
Phenanthroline as a protease inhibitor is a strong chelator. And therefore behaves similar to EDTA.
To address all these issues, our research and development team developed our INDIGO ligand, which has an increased tolerance to EDTA, DTT and phenanthroline during a His-tag purification procedure up to 20 mM each.
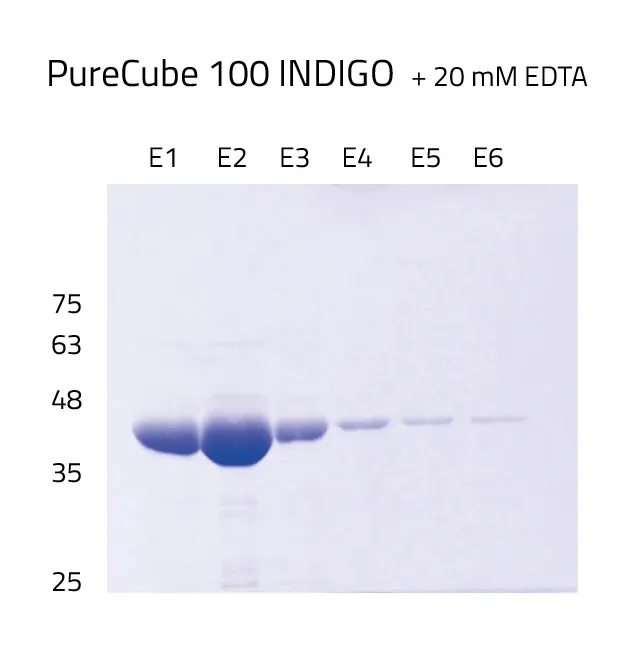
INDIGO's protein purity compared to Ni-NTA
As previously mentioned, His-tag protein purification assays can fall behind in terms of purity compared to other affinity tags. Good examples for this are antibody-affinity tags like FLAG or Rho1D4.
On the flip side, FLAG and Rho1D4 purifications, result in very low protein yields (up to 3-4 mg/ml resin at best) and are better suited for proteins with low abundance.
On the flip side, FLAG and Rho1D4 purifications, result in very low protein yields (up to 3-4 mg/ml resin at best) and are better suited for proteins with low abundance.
His-tag protein purification achieves the highest possible protein yields. During our in-house quality controls for Ni-INDIGO resin we usually obtain around 100 mg protein per ml resin. The protein used for the quality controls is His-tagged GFP.
This video-guide shows His-Tag purification using our His-Affinity beads.
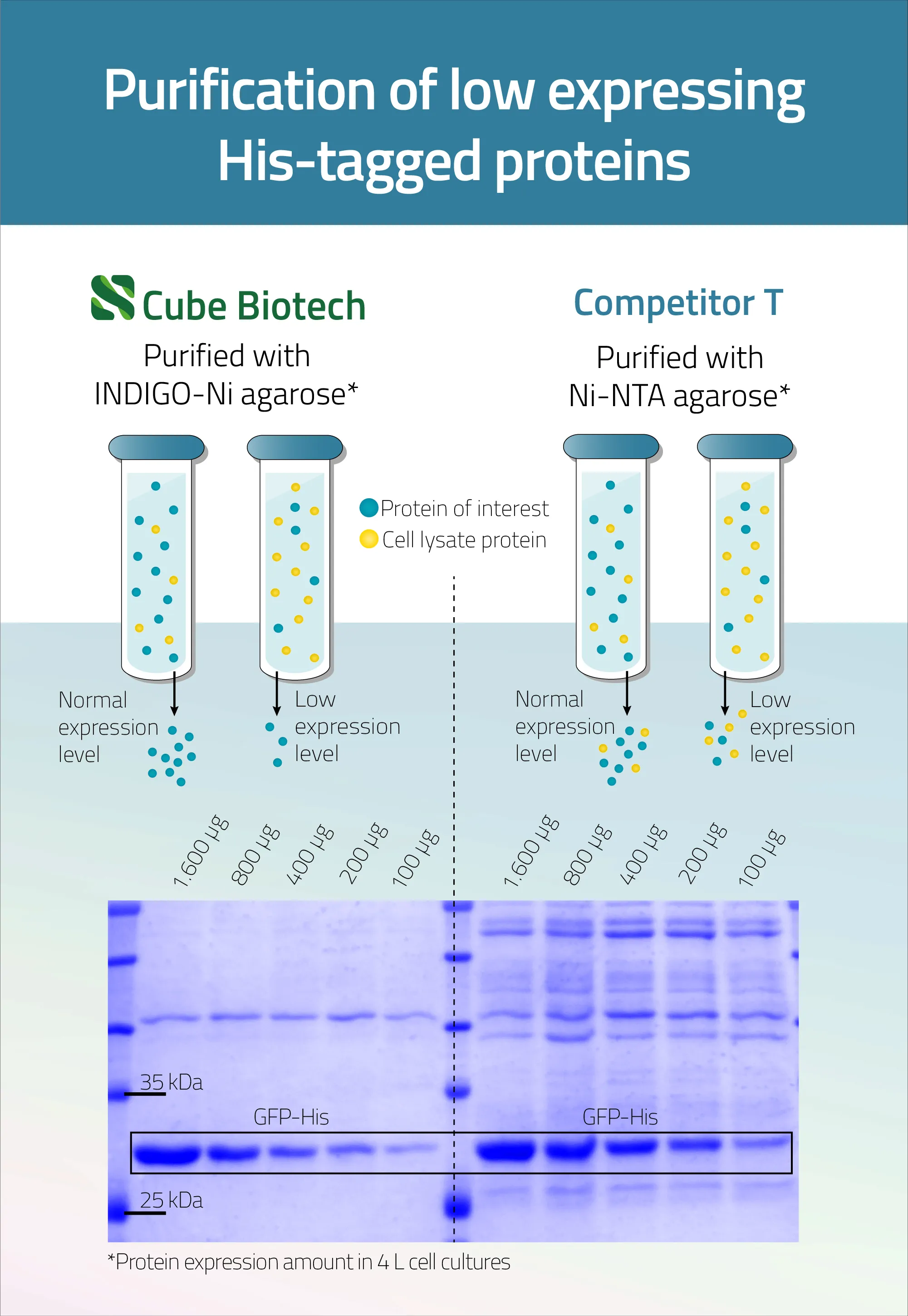
Still not convinced or unsure if you should place a full-scale order just yet?
Just click on the button below and fill out the form to request a 1 mL FREE sample of our 100 Ni-INDIGO agarose.
INDIGO-Ni Products By Cube Biotech
References
- Crowe J, Döbeli H, Gentz R, Hochuli E, Stüber D, Henco K. 6xHis-Ni-NTA chromatography as a superior technique in recombinant protein expression/purification. Methods Mol Biol. 1994;31:371-87. doi: 10.1385/0-89603-258-2:371. PMID: 7921034.
- Day TA, Chen GZ. The metalloprotease inhibitor 1,10-phenanthroline affects Schistosoma mansoni motor activity, egg laying and viability. Parasitology. 1998 Apr;116 ( Pt 4):319-25. doi: 10.1017/s0031182097002370. PMID: 9585934.
- Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. Mechanism of inhibition of proteintyrosine phosphatases by vanadate and pervanadate. J Biol Chem 1997: 272(2): 843–851.
- Scopes RK. Protein purification. New York, NY: Springer New York, 1994.